EXO1-mediated DNA repair by single-strand annealing is essential for BRCA1-deficient cells
Posted on: 15 May 2023 , updated on: 16 May 2023
Preprint posted on 24 February 2023
Categories: molecular biology
Background:
Eukaryotic cells use specialised pathways to repair damaged DNA. Double Strand Breaks (DSBs) are amongst the most dangerous as they compromise both DNA strands. Single Strand Annealing (SSA) is one such pathway used to repair DNA DSBs. This pathway involves resecting (i.e., specifically degrading) sequences flanking the DSB until they form overhangs of single stranded DNA that are homologous in sequence. These two homologous sequences are joined together (‘annealed’) to repair the break.
Though SSA fixes DSBs, it is error prone and results in DNA deletions. BRCA1 is a key factor involved in a high fidelity DSB repair pathway called homologous recombination (HR), playing an important role in tumour suppression. However, BRCA1 may also act during SSA, though what functions BRCA1 may undertake during SSA are still unclear (1). In this preprint, the researchers uncover a relationship between BRCA1-deficiency and a factor involved in resection called EXO1. They show that BRCA1-deficient cells are unable to overcome damage if EXO1 is deleted, suggesting they rely on EXO1’s activities. Their data highlight EXO1 as a potentially important drug target in cancers that are BRCA1-deficient.
Key Findings:
1) In cells lacking BRCA1, long range-resection is important
In a previous screen, the authors found that long-range resection factors were important in BRCA1-deficient cells (2). They selected one of these candidates, the exonuclease EXO1, for further study. By deleting EXO1 in combination with auxin-induced degradation of endogenous BRCA1 tagged with an auxin-inducible degron, they could show that the loss of both factors heavily affected cell growth. This loss of viability was not apparent when BRCA2 was degraded in EXOI-deficient cells. These data suggest that EXO1 is essential when BRCA1 is deficient, but not BRCA2.
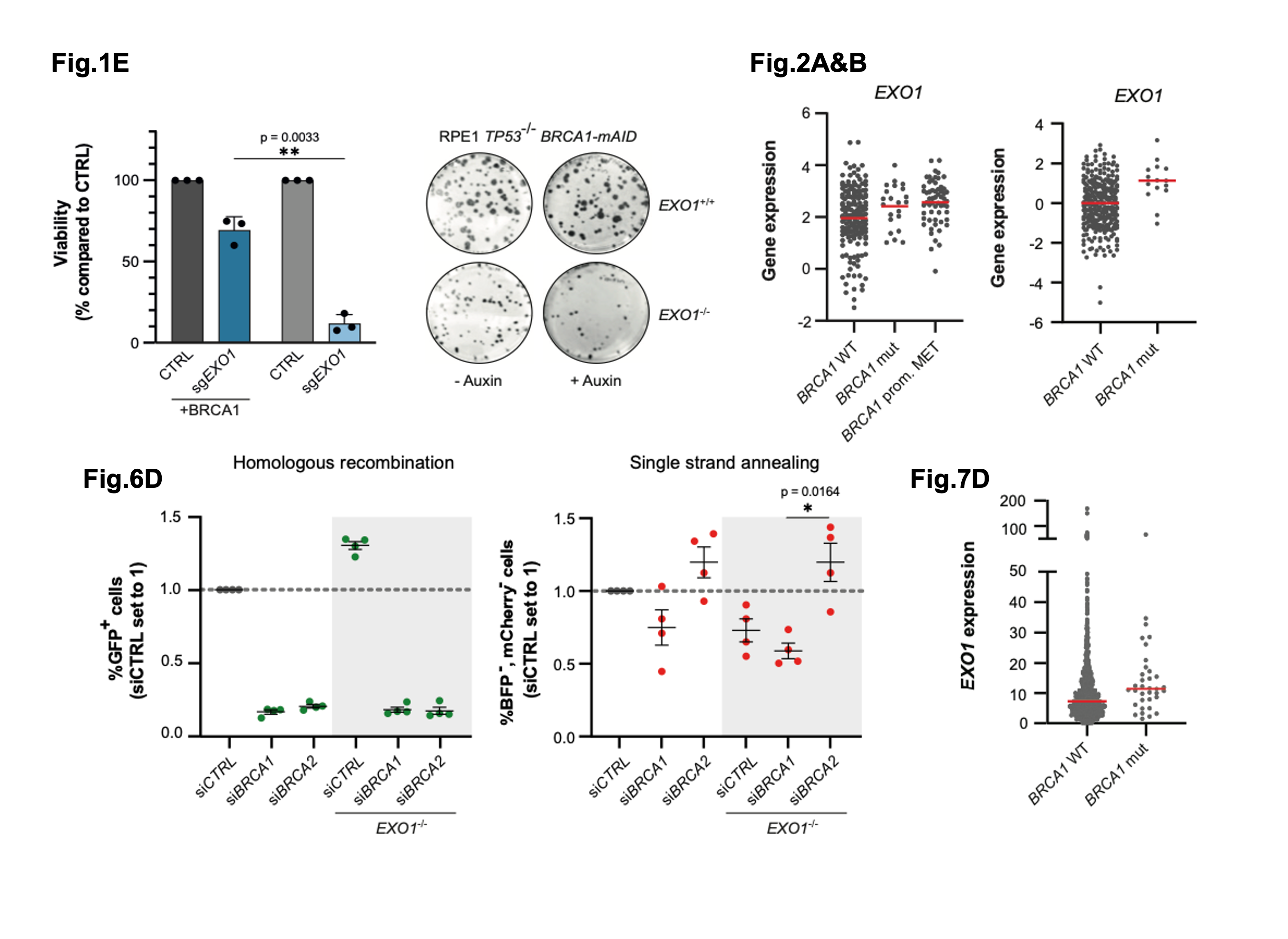
Figure shows a selection of figures from van de Kooij. et al., Figure 1E shows cell viability in the presence or absence of EXO1 when BRCA1 is either present or absent. Figure 2A&B show primary breast cancer tumour samples and the expression of EXO1 in these samples. Figure 6D shows data from an assay that can be used to examine the type of repair pathway choice following a DNA lesion. Figure 7D shows the levels of EXO1 expression across pan-cancer samples in which BRCA1 is mutated or WT. (Adapted from the preprint under a CC-BY 4.0 International License)
2) High EXO1 expression is associated with breast cancer in BRCA1-deficient cells
Using previously published breast cancer datasets, the authors could link increased EXO1 expression to potential defects in HR. HR uses homology sequences to repair a DSB with high fidelity and is less mutagenic than SSA. They concluded that: 1) BRCA1-deficient cells appear to rely on higher levels of EXO1, and 2) EXO1 likely has wider effects in tumours with defects in HR directed repair.
3) When EXO1 is depleted, BRCA1 cells are more unstable
To test if BRCA1 and EXO1 loss, or each factor independently, correlates with genome instability, the authors examined the effects of EXO1 deletion in the absence or presence of BRCA1 depletion. They looked for alterations to the chromosomes, changes to the nuclei of the cells, specifically looking for micronuclei; all of which indicate problems during chromosome segregation or suggest fragmentation of the DNA. They also examined the levels of yH2AX, a phosphorylation event that occurs on the variant histone H2AX (in vertebrates) as a result of genome stress.
When BRCA1 was depleted in the absence of EXO1, a higher number of chromatid aberrations were found suggesting problems during S-phase. This was supported by the observed increase in yH2AX signal in these cells.
4) Loss of EXO1 in BRCA1-deficient cells may lead to ssDNA gaps generated during DNA replication
To understand why BRCA1 is important when EXO1 is lost, the authors deleted a gene called 53BP1. When 53BP1 is deleted in BRCA1-deficient cells, HR is restored, and ssDNA gaps do not form (3-5). However, deletion of 53BP1 does not rescue another issue in BRCA1-deficient cells: replication fork instability (6).
When 53BP1 was deleted, EXO1-depleted BRCA1 KO cells survived. These findings suggest that loss of BRCA1 causes faulty HR or accumulating gaps of ssDNA. The authors then decided to look at the ssDNA gaps by testing for evidence of a post-translational modification called poly(ADP-ribosyl)ation or ‘PAR’, which decorates ssDNA gaps. When EXO1 was lost, but BRCA1 present, the cells accumulated these ssDNA gaps especially during S-phase, however when both EXO1 and BRCA1 were lost, the number of these gaps increased even more, again during S-phase. This suggests that EXO1 is important in preventing these gaps accumulating during S-phase. These lesions can be tolerated under certain conditions (i.e., in the presence of BRCA1 or the loss of BRCA2) but not in BRCA1’s absence.
5) When BRCA1 is deficient, SSA driven by EXO1 is a key repair pathway
Next, the authors asked why EXO1 is important when BRCA1 is deficient. They induced damage by irradiating the BRCA1-deficient cells and looked for RAD51 and RAD52 foci. Foci of RAD51 indicate activation of HR repair, whereas RAD52 foci indicate repair by SSA. They found no additional reduction in RAD51 foci in their BRCA1-deficient cells upon EXO1 depletion (i.e., EXO1 does not affect residual HR in BRCA1-deficient cells) and instead observed a decrease in RAD52 foci. This suggests that these cells likely have problems to repair DSBs by both HR and SSA. Looking more widely at other SSA factors, they found that depletion of other key SSA factors in BRCA1-deficient cells was lethal, like EXO1 loss.
6) BRCA1-deficient cells rely on EXO1 for survival
Using a cell reporter system to examine whether DNA repair has occurred by SSA and/or HR after induction of a DSB, the authors revealed that whilst cells with EXOI deficiency combined with BRCA2 deficiency are unable to effectively undergo HR to resolve DSBs, they can use SSA. However, when EXO1 deficiency was combined with BRCA1 deficiency, cells couldn’t perform HR and they were less able to use SSA.
These data suggest that BRCA1-deficient cells depend on EXO1 to repair DSBs by SSA. In support, they found a higher incidence of SSA use in BRCA1-deficient tumours, and when BRCA2 was deficient, these incidences were even higher.
What I like about this preprint:
Cancer is an extremely difficult disease to treat for many reasons, one of which is the cancer cell’s ability to accumulate mutations and evolve rapidly, thereby increasing the chances of treatment resistance arising. In this preprint, the authors bring into focus a key enzyme (EXO1), which to date has not been widely regarded as a therapeutic target. However, their work herein reveals that EXO1 is important for the survival of cells with deficiencies in the gene BRCA1, a key gene mutated across a multitude of cancers. These new insights may help pave the way for a combined therapy targeting BRCA1-deficient cells by limiting EXO1’s activities.
Questions for the Authors:
Q1: Do increased levels of EXO1 in BRCA1-deficient tumours associate with altered disease progression/severity?
Q2: Are there any EXO1 chemical inhibitors and if so, do you see sensitivity in BRCA1-deficient tumour cells if they are treated with any of these inhibitors?
Q3: Is it possible to speculate as to the pathway preference for DSB repair in BRCA1-deficient cells. i.e., is SSA the preferred repair pathway or is alt-EJ more typical?
Q4: When EXO1 is lost, is there evidence that another long-range section nuclease like BLM could be selected for to rescue the lethality in a BRCA1-deficient background?
References:
- Blasiak, J., Single-Strand Annealing in Cancer. In. J. Mol. Sci, 2021
- Adam, S., Rossi, S.E., Moatti, N., De Marco Zompit, M., Xue, Y., Ng, T.F., Alvarez-Quilon, A., Desjardins, J., Bhaskaran, V., Martino, G., et al. The CIP2A-TOPBP1 axis safeguards chromosome stability and is a synthetic lethal target for BRCA-mutated cancer. Nat Cancer, 2021.
- Bouwman, P., Aly, A., Escandell, J.M., Pieterse, M., Bartkova, J., van der Gulden, H., Hiddingh, S., Thanasoula, M., Kulkarni, A., Yang, Q., et al. 53BP1 loss rescues BRCA1 deficiency and is associated with triple-negative and BRCA-mutated breast cancers. Nat Struct Mol Biol, 2010.
- Bunting, S.F., Callen, E., Wong, N., Chen, H.T., Polato, F., Gunn, A., Bothmer, A., Feldhahn, N., Fernandez-Capetillo, O., Cao, L., et al. 53BP1 inhibits homologous recombination in Brca1- deficient cells by blocking resection of DNA breaks. Cell, 2010.
- Ray Chaudhuri, A., Callen, E., Ding, X., Gogola, E., Duarte, A.A., Lee, J.E., Wong, N., Lafarga, V., Calvo, J.A., Panzarino, N.J., et al. Replication fork stability confers chemoresistance in BRCA- deficient cells. Nature 2016.
doi: https://doi.org/10.1242/prelights.34683
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the molecular biology category:
A drought stress-induced MYB transcription factor regulates pavement cell shape in leaves of European aspen (Populus tremula)
Jeny Jose
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
preLists in the molecular biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |











 (2 votes)
(2 votes)