Liver-Chip: Reproducing Human and Cross-Species Toxicities
Posted on: 7 June 2019 , updated on: 29 September 2019
Preprint posted on 15 May 2019
I’ll have Liver-Chips with that: researchers apply rat, dog, and human liver cells to organ-on-chip technology
Selected by Zhang-He GohCategories: bioengineering, pharmacology and toxicology
Background of preprint
Despite the extensive research into in silico, in vitro, and in vivo toxicity prediction models to date, accurately predicting the toxicity of drugs and toxins remains a scientific and clinical challenge. Indeed, the prediction of hepatotoxicity appears to be a particularly difficult challenge dogged by the twin problems of poor clinical translation and the rarity of idiosyncratic events that only appear as post-marketing signals. Therefore, models with greater predictive power are needed.
To meet this challenge, Jang et al. used human microengineered Organ-on-Chip technology to design species-specific Liver-Chips in their preprint.
Key findings of preprint
The key findings of the preprint by Jang et al. can be categorised into two sections: (A) development and characterisation of Liver-Chips, and (B) investigation and prediction of hepatotoxicity using Liver-Chips.
(A) Development and characterisation of rat, dog, and human Liver-Chips
Jang et al. constructed species-specific Liver-Chips lined by rat, dog, or human hepatic cells (Fig. 1). Primary hepatocytes were used to line the upper parenchymal channel; while liver sinusoidal endothelial cells (LSECs) were used, individually or in conjunction with liver Kupffer cells and stellate cells, to line the opposite side of the same membrane in the lower vascular channel.
By comparing the albumin secretion ability of the dual-cell Liver-Chips (which contained only hepatocytes and LSECs) to hepatocytes cultured alone in static sandwich culture plates, Jang et al. found that higher physiological function was maintained in the case of dual-cell Liver-Chips than in the monocultures. In fact, this function approximated that which was expected from in vitro-in vivo extrapolation (IVIVE).
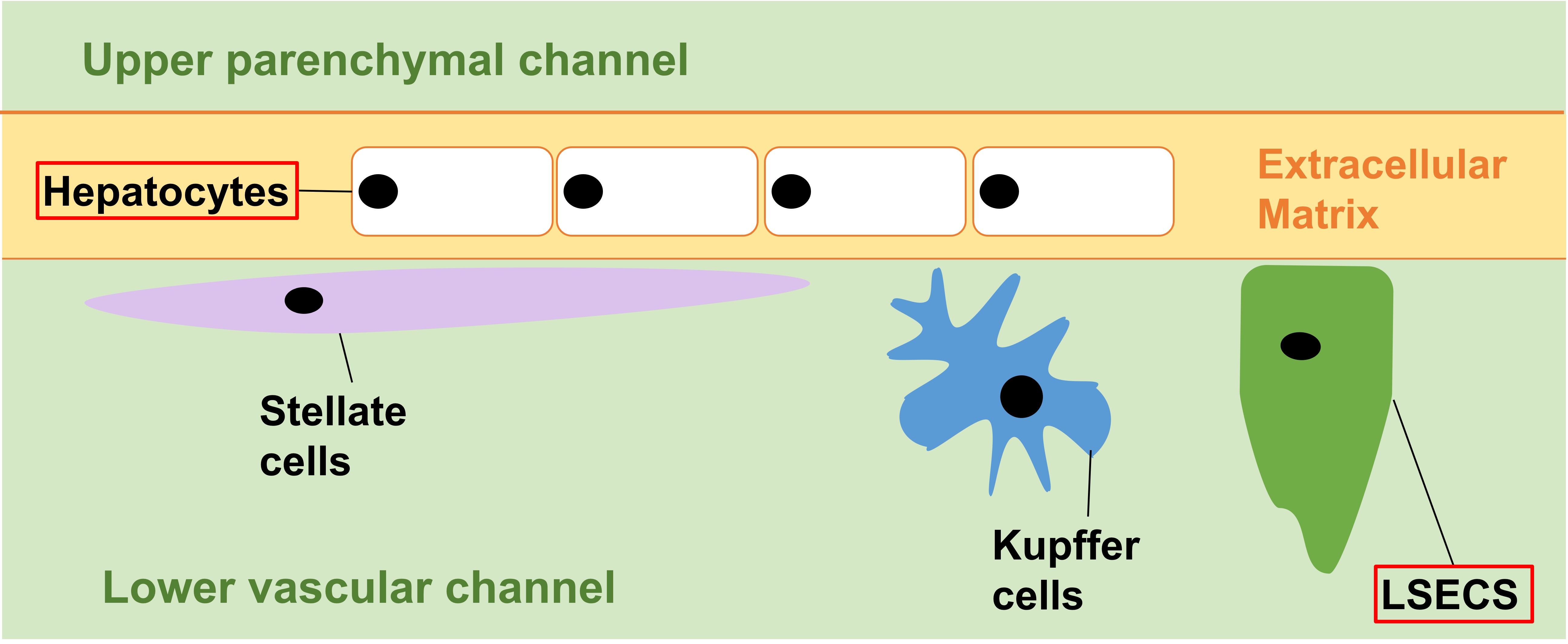
Figure 1. Species-specific Liver-Chips. Cell types in red boxes, hepatocytes and LSECs, were used in dual-cell Liver Chips. All four cell types were used in quadruple-cell Liver-Chips.
The physiological function of dual-cell Liver-Chips was reinforced by the drug metabolising capacity of the hepatocytes over time using CYP1A, CYP2B, and CYP3A. Jang et al. found that while there was a significant decline in CYP activities in rat, dog, and human hepatocytes in sandwich monoculture plates over 14 days, these activities were maintained in the case of dual-cell Liver-Chips.
Jang et al. then tested the dual-cell Liver-Chips’ response to drug-induced liver injury (DILI) using bosentan, a bile salt export pump (BSEP) inhibitor that only affects human hepatocytes. From their results, the authors showed that the dual-cell Liver-Chips could be used to establish links between a toxin’s mechanism of action and its physiological and functional effect on the liver.
(B) Detection and prediction of hepatotoxicity using Liver-Chips
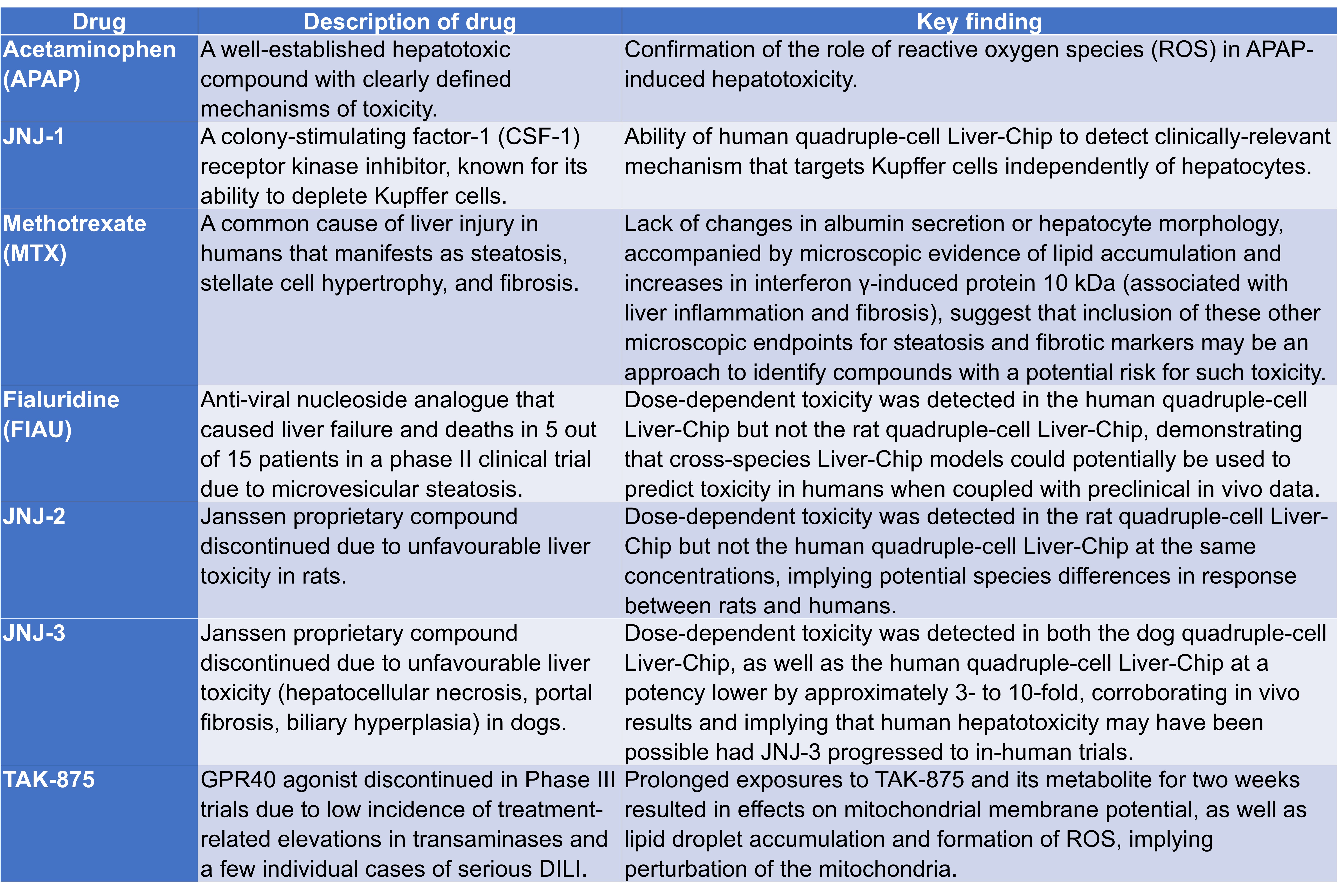
Having established that dual-cell Liver-Chips could be used to investigate mechanisms of drug-induced liver injury (DILI), Jang et al. added species-specific, non-parenchymal cells (i.e. hepatic stellate and Kupffer cells) into the vascular channel, thus producing a quadruple-cell Liver-Chip model. Following characterisation and validation, various drugs were tested for their effects on the quadruple-cell Liver-Chips (Table 1). Specifically, this section includes the modelling of steatosis and identifying markers of fibrosis in Liver-Chips, the use of species-specific Liver-Chips to query human relevance of animal liver toxicities, and the identification of risk for idiosyncratic DILI using the human Liver-Chip.
Table 1. Toxins tested on Liver-Chips and their key findings
Significance of this preprint: Current developments and future directions
In my last preLight, I wrote about the invention of a Bile Duct-on-a-Chip by Du et al. [1]. Similarly, the species-specific Liver-Chip engineered and validated by Jang et al. is an interesting invention that joins researchers’ arsenal of organ-on-chip models available for pharmacology and toxicology research. Indeed, given the anatomic and physiologic proximity between the liver and the bile duct, the descriptions of the miniaturisation of the hepatobiliary systems complement each other.
In this preprint, the authors test the toxicity of different known drugs or toxins on their invention, and show how Liver-Chips could potentially be used to characterise and even predict the toxicities of drugs and their metabolites in preclinical studies. Indeed, the data collected from Liver-Chips may complement preclinical data, aiding researchers and pharmaceutical companies in developing more inexpensive, more rapid, and more accurate methods of detecting the toxicities of drug candidates.
Perhaps the biggest barrier to a more widespread use of organ-on-a-chip technology lies in its regulation. Currently, there is some tentative evidence that the Food and Drug Agency (FDA) may be accepting the use of data generated from organ-on-a-chip technology to complement regulatory submissions [2]. For this reason, I remain cautiously optimistic in believing that organ-on-a-chip technology may come to supplement or even supplant the use of animal in vivo data in the decades ahead.
Questions for authors
It is especially promising that the Liver-Chips were able to detect idiosyncratic DILI responses, as illustrated by TAK-875 in this preprint. This is because there are a few clinically-approved drugs that are also known to give rise to idiosyncratic DILI, especially antimicrobials such as tetracycline antibiotics, antitubercular agents, and anti-HIV antivirals. What challenges do you foresee in using the Liver-Chip model to predict or investigate the hepatotoxicity of these other agents, and what modifications to the testing procedures might be needed to overcome these difficulties?
References
[1] Du Y, Khandekar G, Llewellyn J, Polacheck W, Chen CS, Wells RG, A Bile Duct-on-a-Chip with Organ-Level Functions, bioRxiv (2019) 594291.
[2] Isoherranen N, Madabushi R, Huang S-M, Emerging Role of Organ-on-a-Chip Technologies in Quantitative Clinical Pharmacology Evaluation, Clinical and Translational Science 12(2) (2019) 113-121.
doi: https://doi.org/10.1242/prelights.11122
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the bioengineering category:
A Novel Chimeric Antigen Receptor (CAR) - Strategy to Target EGFRVIII-Mutated Glioblastoma Cells via Macrophages
Dina Kabbara
Human pluripotent stem cell-derived macrophages modify development of human kidney organoids
Theodora Stougiannou
Matrix viscoelasticity regulates dendritic cell migration and immune priming
Roberto Amadio
Also in the pharmacology and toxicology category:
Snake venom metalloproteinases are predominantly responsible for the cytotoxic effects of certain African viper venoms
Daniel Osorno Valencia
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
In vitro pharmacokinetics and pharmacodynamics of the diarylquinoline TBAJ-587 and its metabolites against Mycobacterium tuberculosis
Zhang-He Goh
preLists in the bioengineering category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Advances in microscopy
This preList highlights exciting unpublished preprint articles describing advances in microscopy with a focus on light-sheet microscopy.
| List by | Stephan Daetwyler |
Also in the pharmacology and toxicology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
COVID-19 / SARS-CoV-2 preprints
List of important preprints dealing with the ongoing coronavirus outbreak. See http://covidpreprints.com for additional resources and timeline, and https://connect.biorxiv.org/relate/content/181 for full list of bioRxiv and medRxiv preprints on this topic
| List by | Dey Lab, Zhang-He Goh |
1
Drug use in special populations
Any drugs that are being used in special populations: Patients with liver and kidney failure, in paediatrics, in geriatrics, and in pregnant or lactating patients. Includes the discovery of factors that could potentially affect drug use in these special populations.
| List by | Zhang-He Goh |
Toxicology of toxicants, existing therapeutics, and investigational drugs
Preprints that describe the toxicology of environmental pollutants and existing and upcoming drugs. Includes both toxicokinetics and toxicodynamics, as well as technological improvements that will help in the characterisation of this field.
| List by | Zhang-He Goh |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Anticancer agents: Discovery and clinical use
Preprints that describe the discovery of anticancer agents and their clinical use. Includes both small molecules and macromolecules like biologics.
| List by | Zhang-He Goh |
Advances in Drug Delivery
Advances in formulation technology or targeted delivery methods that describe or develop the distribution of small molecules or large macromolecules to specific parts of the body.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)