Sex-specific topology of the nociceptive circuit shapes dimorphic behavior in C. elegans
Posted on: 23 December 2021
Preprint posted on 15 December 2021
Article now published in Current Biology at http://dx.doi.org/10.1016/j.cub.2022.08.038
Rewiring a single synaptic connection reprograms a sexually dimorphic behavior in C. elegans.
Selected by Chee Kiang EweCategories: animal behavior and cognition, molecular biology, neuroscience
Background:
Sexual selection often results in sexual dimorphism – the differences in appearance and behavior between males and females of the same species – that promotes reproductive success. For example, in many species of songbirds, the male produces a complex song to attract mating partners, whereas the female does not. However, this sexual display may unwittingly lead to increased exposure to predators and parasites. This “viability cost” is optimized during evolution to ensure the overall fitness of the species in different environments [1,2].
Despite a relatively simple nervous system (302 and 387 neurons in hermaphrodite and male, respectively), the nematode C. elegans exhibits many complex sex-specific behaviors. The recent reconstruction of the connectomes of both male and hermaphrodite worms provides a springboard for understanding the neuronal basis of sexually dimorphic characteristics [3]. It has previously been shown in C. elegans that sex-specific neurons may drive sexually dimorphic behaviors. For example, the distinct pheromone response between sexes can be, in part, attributed to the head CEM sensory neurons present only in males [4]. In other cases, the variation in synaptic wiring patterns among the sex-shared neurons can generate dimorphic outputs. In this fascinating preprint, the authors demonstrated that dimorphic behavioral responses evoked by noxious/painful sensations (known as nociception) in C. elegans is driven by the same set of sex-shared neurons that are differentially wired, providing an important insight into the molecular mechanism underlying sex-specific circuit development.
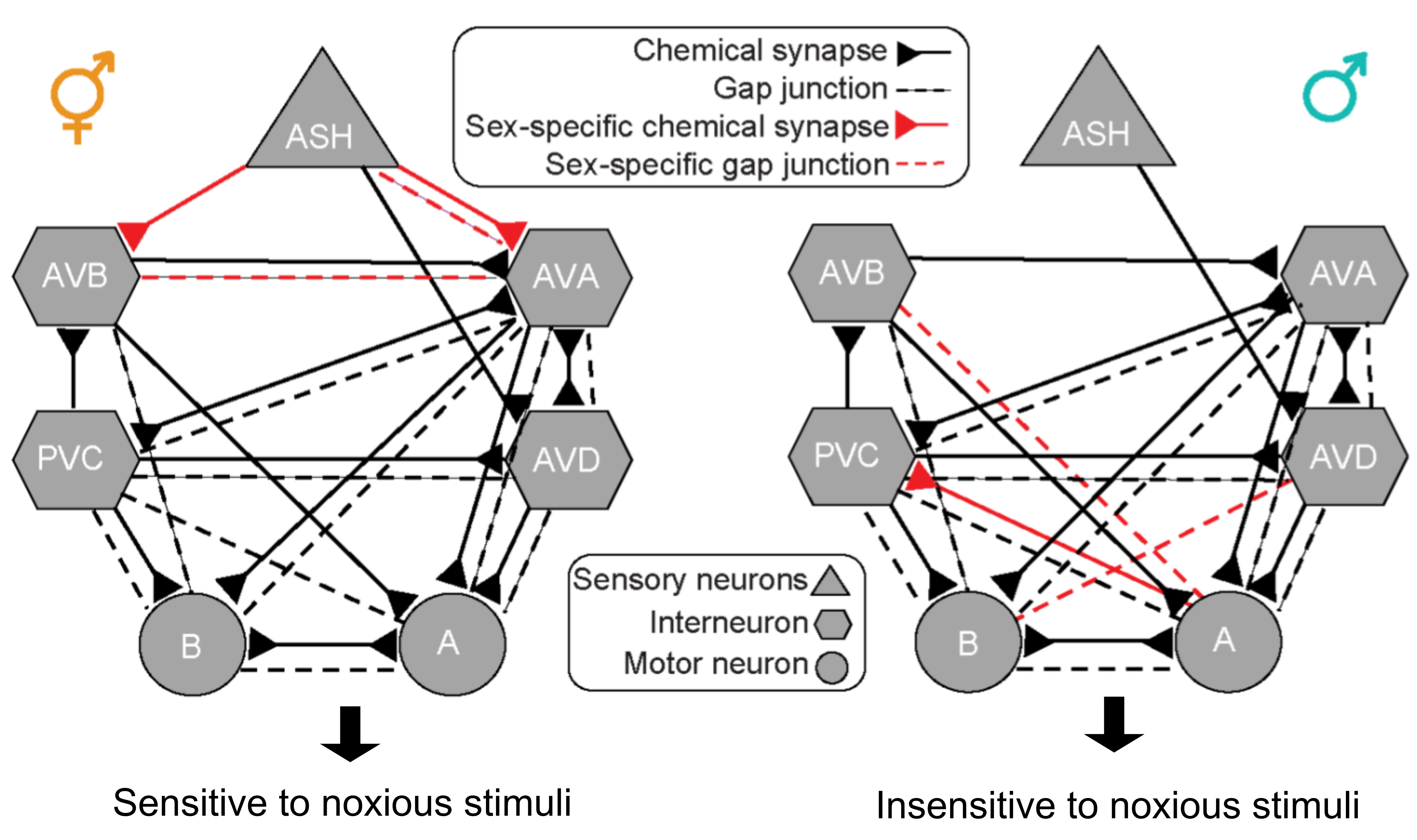
Figure 1: Hermaphrodite (left) vs. male (right) nociceptive circuit. The distinct connectivity structures give rise to sexually dimorphic behaviors (adapted from figures 2; Pechuk, Goldman, Salzberg, et al. 2021).
Main findings:
- C. elegans exhibits sexually dimorphic aversive behaviors
To investigate sex-specific aversion response, the authors exposed both males and hermaphrodites to various noxious stimuli, including SDS, glycerol, quinine, and copper. While both sexes avoided noxious stimuli at high concentrations, the authors found that hermaphrodites showed an increased sensitivity at low concentrations.
- Variation in the connectivity between the sex-shared neurons generates sexually dimorphic behaviors
ASH neurons, the primary nociceptors in C. elegans, relay sensory information through the downstream interneurons that mediate forward (AVB, PVC) and backward (AVA, AVD) movement. Although all the neurons in this circuit are sex-shared, the synaptic wiring patterns are sexually dimorphic. For example, ASH forms chemical synapses with AVA and AVB only in hermaphrodites, not in males (Figure 1).
The authors found that activating ASH neurons (which were modified to contain a light-sensitive ion channel) with LED light triggered sexually dimorphic aversive behaviors; however, sensory transduction in ASH did not appear to exhibit dimorphism as the expression of sensory receptors and their downstream signaling molecules, calcium response, and the synaptic transmission machinery were similar in both sexes. The authors later showed that, in contrast, sex differences in the connectivity in the nociceptive circuit (downstream of ASH input) could account for the distinct behavioral output.
To investigate how the neural network structure might give rise to dimorphic behavior, the authors performed computational simulation and found a small set of biophysical parameters that would trigger an aversive response upon ASH activation in both males and hermaphrodites, as observed experimentally. Importantly, the same parameters were shown to activate dimorphic behaviors in male and hermaphrodite with different circuit structures.
Using calcium imaging, AVA interneurons were found to behave differently in males and hermaphrodites in response to aversive stimuli as the calcium response was stronger and lasted longer in hermaphrodites than in males. Male ASH neurons do not connect to AVA (Figure 1), but feminizing sensory neurons by expressing TRA-2 (driven by osm-5 promotor that is activated in most ciliated sensory neurons), which stabilizes the TRA-1 hermaphroditic identity determinant transcription factor in otherwise male animals [5], causes ectopic formation of ASH-AVA synapses as well as hermaphrodite-like nociceptive response. Remarkably, artificially tethering ASH and AVA by expressing mammalian connexin36-mediated synthetic gap junctions in the two neurons in males promoted glycerol-induced aversive behavior, as observed in wild-type hermaphrodites. Thus, sexually dimorphic ASH-AVA connection gives rise to distinct nociceptive behaviors in males and hermaphrodites.
Curiously, masculinizing the hermaphrodite nervous system showed very little effect on synaptic patterning and behaviors, suggesting that the hermaphrodite nervous system is more robust than that of males, which may have important evolutionary and ecological implications.
- Rewired males are less efficient at searching for hermaphrodites
To investigate whether the dimorphic nociceptive neuronal circuit controls other behavioral outputs outside of nociception, the authors turned their attention to mate searching and mating behaviors which are also highly sex-specific. The authors activated ASH with light in wild-type and sensory-feminized males and compared their ability to search and contact the hermaphrodites while receiving optogenetic nociceptive stimulations. It was found that sensory-feminized males are less efficient at reaching the hermaphrodites, suggesting that the altered circuitry topology that allows males to readily avoid noxious stimuli impairs mating behaviors. Hence, the sexually dimorphic nociceptive circuit is likely the consequence of sexual selection that promotes male mating success.
What I liked about this preprint:
Using a network model, the authors predicted how sex-specific synapses generate dimorphic behaviors and confirmed their findings through a series of elegant molecular analyses. The finding that sexually dimorphic behavioral response can be tuned by rewiring a single synaptic connection is particularly striking!
Questions to the authors:
How do you think the nociceptive circuit might interact with the mating circuit?
Can sensory-feminized males still respond to mate-finding pheromone cues from the hermaphrodites?
References:
- Okada K, Katsuki M, Sharma MD, Kiyose K, Seko T, Okada Y, et al. Natural selection increases female fitness by reversing the exaggeration of a male sexually selected trait. Nature Communications 2021 12:1. 2021;12: 1–10. doi:10.1038/s41467-021-23804-7
- Møller AP, Nielsen JT, Garamszegi LZ. Song post exposure, song features, and predation risk. Behavioral Ecology. 2006;17: 155–163. doi:10.1093/BEHECO/ARJ010
- Cook SJ, Jarrell TA, Brittin CA, Wang Y, Bloniarz AE, Yakovlev MA, et al. Whole-animal connectomes of both Caenorhabditis elegans sexes. Nature. 2019;571: 63–71. doi:10.1038/s41586-019-1352-7
- Narayan A, Venkatachalam V, Durak O, Reilly DK, Bose N, Schroeder FC, et al. Contrasting responses within a single neuron class enable sex-specific attraction in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 2016;113: E1392–E1401. doi:10.1073/PNAS.1600786113/-/DCSUPPLEMENTAL
- Mowrey WR, Bennett JR, Portman DS. Distributed Effects of Biological Sex Define Sex-Typical Motor Behavior in Caenorhabditis elegans. The Journal of Neuroscience. 2014;34: 1579. doi:10.1523/JNEUROSCI.4352-13.2014
doi: https://doi.org/10.1242/prelights.31197
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the animal behavior and cognition category:
Cannibalism as a mechanism to offset reproductive costs in three-spined sticklebacks
Tina Nguyen
Morphological variations in external genitalia do not explain the interspecific reproductive isolation in Nasonia species complex (Hymenoptera: Pteromalidae)
Stefan Friedrich Wirth
Trade-offs between surviving and thriving: A careful balance of physiological limitations and reproductive effort under thermal stress
Tshepiso Majelantle
Also in the molecular biology category:
A drought stress-induced MYB transcription factor regulates pavement cell shape in leaves of European aspen (Populus tremula)
Jeny Jose
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
Also in the neuroscience category:
Electrophysiological correlates of conscious experiences during sleep: Lucid dreams, sleep paralysis, out-of-body experiences, and false awakenings
uMontreal Neuro preLighters et al.
PPARδ activation in microglia drives a transcriptional response that primes phagocytic function while countering inflammatory activation
Isabel Paine
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
preLists in the animal behavior and cognition category:
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Bats
A list of preprints dealing with the ecology, evolution and behavior of bats
| List by | Baheerathan Murugavel |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Also in the molecular biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Also in the neuroscience category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |











 (No Ratings Yet)
(No Ratings Yet)