Synergistic and independent roles for Nodal and FGF in zebrafish CPC migration and asymmetric heart morphogenesis
Posted on: 24 July 2025 , updated on: 31 October 2025
Preprint posted on 18 May 2025
Article now published in Development at http://dx.doi.org/10.1242/dev.204873
‘These signals, they’re made for jogging’: The authors of this study find that Nodal and FGF signals regulate early zebrafish cardiac morphogenetic events and lead to asymmetry in migration patterns that result in cardiac jogging and cardiac looping.
Selected by Theodora StougiannouCategories: developmental biology
Updated 31 October 2025 with a postLight by Theodora M Stougiannou
This study by Gonzalez and team has now been published in Development. When comparing the preprint uploaded to BioRxiv on May 18, 2025 with the peer-reviewed paper, one main difference in content can be observed. This revolves around the relationship between FGF, F-actin and the resulting migration of cardiac progenitors during cardiac jogging.
Cardiac jogging describes the process during which migration of cardiac progenitors follows a leftward and anterior motion, with progenitors on the left migrating faster than progenitors on the right.
Though initially both FGF and Nodal were described as contributors to F-actin levels in migrating left atrial cells, results included in the final version of the paper show that only Nodal induced an increase in F-actin in these left atrial cell groups, something that was not seen with FGF. As a result of this effect of Nodal on these populations, left atrial cells migrate faster than their counterparts on the right, driving clockwise rotation of the cone. This assertion is further substantiated with results from experiments measuring mean fluorescence intensity differences between wild-type zebrafish embryos and zebrafish embryos treated with SU5402, an inhibitor of FGF. Measured populations included not only migrating left versus right atrial cardiac progenitors, but the entire cardiac cone as well [“We also measured the mean fluorescence intensity….laterality”, page 7]. These results show that the abundance of F-actin in the cardiac cone, overall, is not affected by disruptions in FGF signaling. Thus, while FGF acts permissively to promote motility of cardiac progenitors, Nodal acts instructively to specifically direct the laterality of these migrating progenitors. This change is further reflected in one of the Figures included in the final version of the preprint as well as the published manuscript, noting that only Nodal affects F-actin levels in left migrating atrial progenitors.
It is important to note that the alteration described above was already evident during the writing of the preLight, as the authors described this change in results during the interview. The published manuscript includes an additional explanation for this observation. Overall, this highlights the importance of constructive peer review, as well as the importance of revisiting experimental results to further add to the knowledge of a phenomenon being studied at the time; all critical components in the pursuit of truth.
‘These signals, they’re made for jogging’: Nodal and FGF signals regulate early zebrafish cardiac morphogenesis, lead to asymmetry in migration patterns, contribute to cardiac jogging and cardiac looping.
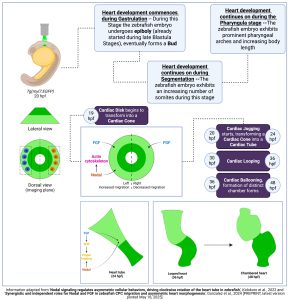
Figure 1 ‘Synergistic and independent roles for Nodal and FGF in zebrafish CPC migration and asymmetric heart morphogenesis’ Study Summary
Background: The zebrafish (Danio rerio), member of the teleost class, e.g., fish with a bony skeleton (as opposed to chondrichthyes, e.g., sharks and rays which possess a cartilaginous skeleton) [1] [2] is an important model organism for studying developmental mechanisms, including development of the heart. As opposed to other mammals, zebrafish development is rapid and external with many of the embryonic and extraembryonic membranes exhibiting transparent morphology, facilitating study and observation. The adult zebrafish heart consists of the sinus venosus (SV), the posterior-inferior placed atrium (A), the anteriorly placed ventricle (V) and the superiorly placed bulbus arteriosus (BA) [3]. Evolutionary conservation in developmental mechanisms between zebrafish and higher mammals, including humans, allows researchers to use zebrafish for the study of developmental processes that can be observed in humans as well.
Zebrafish development is usually annotated as hours post-fertilization (hpf) or days post-fertilization (dpf). It comprises several embryonic stages occurring after external egg fertilisation; completion of embryonic development and subsequent hatching from the egg enclosure occurs around 72 hpf. However, this timeline may change dependent on the thickness of the surrounding membranes. At this stage, the embryo now becomes a larva, and growth continues with the help of yolk-derived nutrients, for about 3 days. At this point, the animal can be considered a juvenile, a stage that lasts for up to the 90 dpf timepoint and at which point, animals are considered adults [4]. Heart development commences around the period of gastrulation, a period characterised by emergence of the embryonic germ layers, ectoderm derived from the epiblast and mesoderm along with endoderm, derived from mesendoderm, all layers originating from the initial blastoderm [5].
In zebrafish, the heart undergoes several distinct morphological stages; in addition, it exhibits left-right asymmetry, driven by molecular networks which are evolutionarily conserved between different organisms [6].
Table 1 Characteristic morphogenetic stages in zebrafish heart development. CPC, Cardiac progenitor cell; LPM, Lateral plate mesoderm; SHF, Second heart field; hpf, hours post fertilization; FGF, Fibroblast growth factor.
| Epithelial Sheets – When: Gastrulation | CPC present bilaterally in the LPM of the developing zebrafish embryo |
| Cardiac Cone/Heart tube – When: Segmentation (21 to 25-Somite Stage) | CPC sheets converge medially, towards the embryonic midline and form a disk (19.5 hpf) which then forms a cardiac cone (21.5 hpf) that eventually extends into a tube (28 hpf) [7]
Cardiac jogging: CPC migration follows a leftward and anterior motion, with progenitors on the left migrating faster than progenitors on the right – FGF, Nodal signals cooperate to regulate heart tube length after jogging (24 hpf) |
| Heart tube elongation – When: Pharyngula (24-30 hpf) | SHF populations are added to either pole of the heart tube (24-48 hpf), contributing to elongation and outflow tract formation |
| Cardiac looping – When: Pharyngula (30-48 hpf) | Heart tube loops towards the right, chamber formation and alignment start to occur; at the end of cardiac looping atrium and ventricle are positioned next to each other |
The authors of the study use zebrafish embryos to characterise the cooperation between Nodal and FGF signalling during early heart development.
Key results of the study:
- Nodal and FGF signals act synergistically during heart development; Nodal upregulates FGF signaling components, although FGF does not directly participate in the establishment of left–right (L-R) heart asymmetry (any FGF signaling effect must occur downstream of L-R axis development) or laterality during cardiac jogging. However, both Nodal and FGF signaling are implicated in CPC migration during this process.
- Nodal and FGF signals regulate the direction and velocity of migrating CPC during cardiac jogging; effects from these signals are additive. Regarding trajectory direction, both Nodal and FGF contribute to anterior bound migration. However, only asymmetric Nodal signals contribute to lateral migration and in particular, left bound migration. On the other hand, FGF acts as a permissive factor in cell migration and further cooperates with Nodal signaling to regulate overall CPC migration.
- While loss of either Nodal, FGF reduces velocity of migration during cardiac jogging, loss of both severely disrupts CPC migration and even formation of the heart tube itself.
- Nodal signals affect the dynamic polymerization and depolymerization events that underpin CPC migration; the asymmetry in Nodal signaling results in asymmetry in F-actin formation and as a result, asymmetry during atrial CPC migration. In particular, Nodal signaling induces an increase in F-actin in migrating left atrial CPC only; as a result, these cells migrate faster than their right-sided counterparts.
- Nodal, FGF signals both promote cardiac looping; FGF also promotes addition of SHF populations to the arterial pole of the zebrafish heart tube with absence of FGF leading to shorter heart tubes [8].
Why this work is interesting:
This study highlights the differential effects on cellular migration and velocities stemming from differential Nodal activity, which in turn cooperates with FGF regulates cardiac jogging. Cardiac jogging is the first instance of L-R asymmetry during heart development. Later down the line, Nodal and FGF also cooperate to regulate another event of L-R asymmetry, heart looping. It is collective cell migrations such as these, which differ across the L-R axes, that contribute to the complex heart forms of vertebrate organisms.
References:
[1] Witten PE, Harris MP, Huysseune A, Winkler C. Chapter 13 – Small teleost fish provide new insights into human skeletal diseases. In: Detrich HW, Westerfield M, Zon LI, editors. Methods in Cell Biology, vol. 138, Academic Press; 2017, p. 321–46. https://doi.org/10.1016/bs.mcb.2016.09.001.
[2] Stedman NL, Garner MM. Chapter 40 – Chondrichthyes. In: Terio KA, McAloose D, Leger JSt, editors. Pathology of Wildlife and Zoo Animals, Academic Press; 2018, p. 1003–18. https://doi.org/10.1016/B978-0-12-805306-5.00040-7.
[3] Sedmera D, Reckova M, deAlmeida A, Sedmerova M, Biermann M, Volejnik J, et al. Functional and morphological evidence for a ventricular conduction system in zebrafish and Xenopus hearts. American Journal of Physiology-Heart and Circulatory Physiology 2003;284:H1152–60. https://doi.org/10.1152/ajpheart.00870.2002.
[4] Singleman C, Holtzman NG. Growth and Maturation in the Zebrafish, Danio Rerio: A Staging Tool for Teaching and Research. Zebrafish 2014;11:396–406. https://doi.org/10.1089/zeb.2014.0976.
[5] Shah G, Thierbach K, Schmid B, Waschke J, Reade A, Hlawitschka M, et al. Multi-scale imaging and analysis identify pan-embryo cell dynamics of germlayer formation in zebrafish. Nat Commun 2019;10:5753. https://doi.org/10.1038/s41467-019-13625-0.
[6] Desgrange A, Le Garrec J-F, Meilhac SM. Left-right asymmetry in heart development and disease: forming the right loop. Development 2018;145:dev162776. https://doi.org/10.1242/dev.162776.
[7] Kidokoro H, Saijoh Y, Schoenwolf GC. Nodal signaling regulates asymmetric cellular behaviors, driving clockwise rotation of the heart tube in zebrafish. Commun Biol 2022;5:996. https://doi.org/10.1038/s42003-022-03826-7.
[8] Gonzalez V, Grant MG, Suzuki M, Christophers B, Williams JR, Burdine RD. Synergistic and independent roles for Nodal and FGF in zebrafish CPC migration and asymmetric heart morphogenesis 2025:2024.01.05.574380. https://doi.org/10.1101/2024.01.05.574380.
[9] Wolton M, Davey MG, Dietrich S. At early stages of heart development, the first and second heart fields are a continuum of lateral head mesoderm-derived, cardiogenic cells. Developmental Biology 2025;520:200–23. https://doi.org/10.1016/j.ydbio.2025.01.009.
doi: https://doi.org/10.1242/prelights.41108
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the developmental biology category:
Cooperation between cortical and cytoplasmic forces shapes planar 4-cell stage embryos
Corentin Mollier, Shivani Dharmadhikari
A drought stress-induced MYB transcription factor regulates pavement cell shape in leaves of European aspen (Populus tremula)
Jeny Jose
Cross Sectional and Longitudinal Imaging Reveals Spatiotemporal Divergence in Morphogenesis and Cell Lineage Specification between in-vivo and in-vitro Mouse Embryo during Pre- and Peri-implantation
Heather Pollington
preLists in the developmental biology category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
BSDB/GenSoc Spring Meeting 2024
A list of preprints highlighted at the British Society for Developmental Biology and Genetics Society joint Spring meeting 2024 at Warwick, UK.
| List by | Joyce Yu, Katherine Brown |
GfE/ DSDB meeting 2024
This preList highlights the preprints discussed at the 2024 joint German and Dutch developmental biology societies meeting that took place in March 2024 in Osnabrück, Germany.
| List by | Joyce Yu |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
2nd Conference of the Visegrád Group Society for Developmental Biology
Preprints from the 2nd Conference of the Visegrád Group Society for Developmental Biology (2-5 September, 2021, Szeged, Hungary)
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Society for Developmental Biology 79th Annual Meeting
Preprints at SDB 2020
| List by | Irepan Salvador-Martinez, Martin Estermann |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EDBC Alicante 2019
Preprints presented at the European Developmental Biology Congress (EDBC) in Alicante, October 23-26 2019.
| List by | Sergio Menchero et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |











 (No Ratings Yet)
(No Ratings Yet)