The Drosophila anterior-posterior axis is polarized by asymmetric myosin activation
Posted on: 9 June 2021 , updated on: 10 June 2021
Preprint posted on 21 April 2021
Article now published in Current Biology at http://dx.doi.org/10.1016/j.cub.2021.11.024
The silent conductor of cortical polarisation: asymmetric activation of Myosin-II orchestrates A/P axis establishment in Drosophila
Selected by Giuliana ClementeCategories: developmental biology
Context and Background:
The formation of the main body axis is a tightly regulated process, fundamental for successful embryonic development. In Drosophila, body axis patterning is established earlier on during oogenesis, well before egg fertilization and deposition. The process requires a complex cross talk between the oocyte and the surrounding somatic epithelium, and it is guided by multiple hierarchical steps of symmetry-breaking events within the oocyte. The first polarising signal is detectable at stage 6-7 of egg development (Fig.1). At this stage, the release of the morphogen Gurken by the oocyte induces the follicle cells to commit towards a posterior fate through the activation of the EGF-like receptor Torpedo [1] (Fig.1, step1, red arrows). The posterior follicle cells induce the establishment of cortical polarity in the oocyte, sending back a signal of which the molecular nature is still unknown. (Fig.1, step1, green arrows). Under the influence of this signal, the oocyte recruits the polarity protein PAR-1 to the posterior cortex in an actin-dependent manner [2, 3] (Fig.1, step2, green crescent). Meanwhile, aPKC and Par-6 are actively excluded from the posterior cortex and recruited and maintained at the anterior (Fig.1, step 2, purple crescent). The establishment of cortical polarity is reinforced by mutual exclusion between the anterior and posterior PAR complexes. This series of events leads to the polarisation of the oocyte microtubule network (Fig.1, step 3) and the consequent asymmetric distribution of cell fate determinants (Fig.1, step 4). Additionally, the oocyte nucleus (Fig.1, step3, in orange) migrates to a more antero-dorsal position as a consequence of microtubule polarisation, setting the basis for the formation of the dorsal-ventral (D/V) axis of the future embryo.
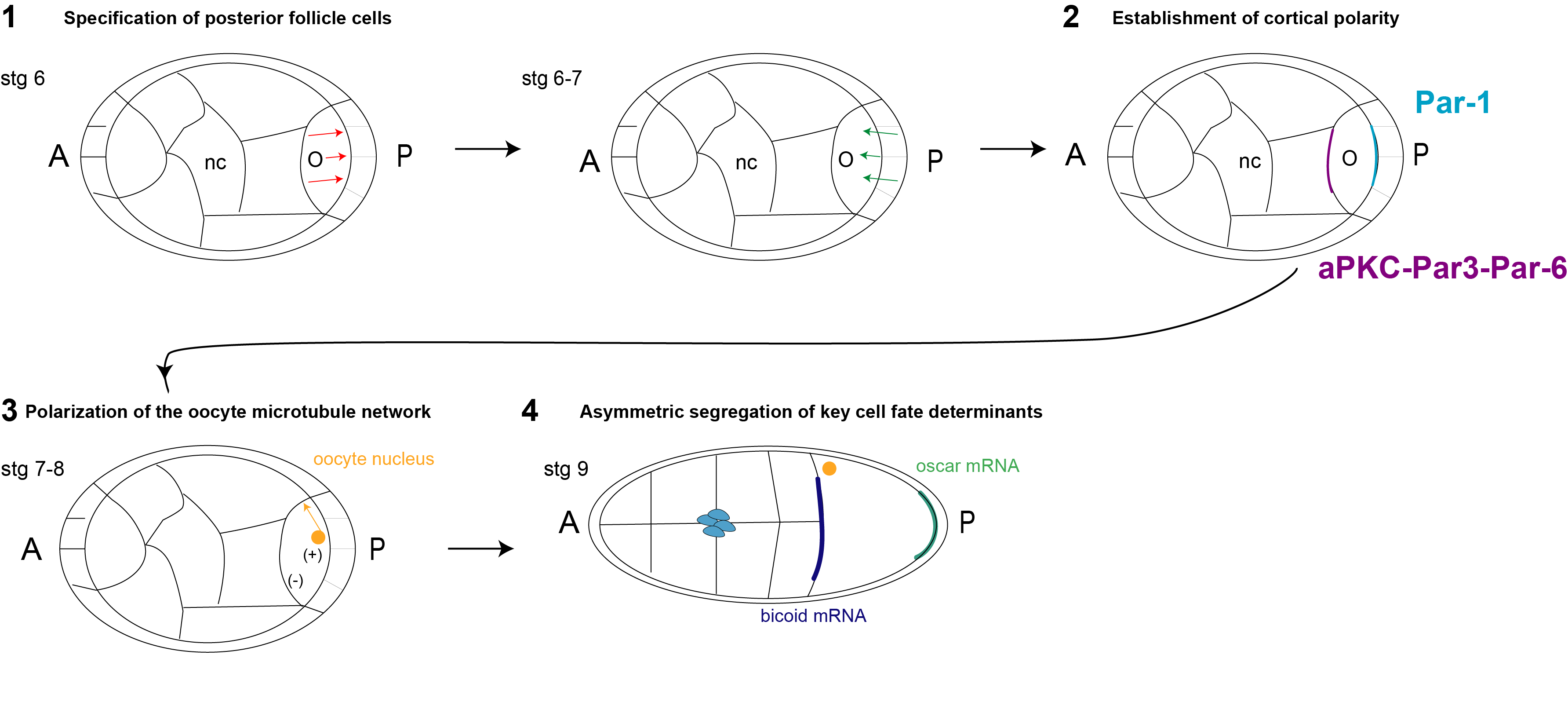
Figure 1: Schematic summary of the main steps leading to the establishment of A/P and D/V polarity in Drosophila.
In this preprint, Doerflinger et al. investigate how the signal behind the establishment of oocyte cortical polarity is transduced at a molecular level and identify the first event of polarity establishment in the asymmetric activation of non-muscle Myosin II.
Main findings:
By performing a germline clone screen for mutants with disrupted A/P polarity, the authors identified a complementation group of three mutant alleles which showed clear signs of compromised anterior-posterior polarization: oocyte microtubule network failed to polarize, PAR-1 crescent at the posterior end failed to form and the oocyte nucleus at stage 10 of egg development failed to occupy the classical antero-lateral localization. The mutant alleles were mapped to the unc-45 locus which encodes for a chaperone protein known to fold and stabilize myosins in general and specifically non-muscle Myosin-II (Myo-II) in this particular context. Therefore, they explored whether activation of non-muscle Myo-II was required for establishment of polarity. They found that the di-phosphorylated active form of Myo-II was enriched in a posterior crescent, from stage 7 of egg chamber development onwards. Activation of Myo-II was dependent on the polarising signal Gurken but was upstream of Par-1 cortical recruitment. Moreover, the authors showed that di-phosphorylation of Myosin regulatory light chain (known as Spaghetti squah ‘sqh’ in flies) was required for the polarisation of the oocyte. Indeed, 50% of sqh null mutantant egg chambers overexpressing a mutant form of Sqh in which threonine 20 was mutated into alanine (sqhAS) failed to localise Par-1 at the posterior cortex and consequently, to transport Staufen to the posterior pole of the oocyte.
Were Myo-II activation and cortical contraction continuously required to maintain polarisation? Pharmacological treatment of egg chambers with ML-7, an inhibitor of myosin light chain kinase, resulted in the fast and complete loss of di-phosphorylated Myo-II and Par-1 from the posterior cortex, confirming the central role played by Myo-II phosphorylation in the establishment and maintenance of polarity.
Relevance:
This work provides the first evidence that di-phosphorylation of Myo-II is required for the establishment and maintenance of cortical polarity in the Drosophila oocyte. Preventing myosin-II activation disrupts the recruitment of Par-1 to the posterior cortex and compromises axis formation. The Myo-II dependent localisation of Par-1 in the oocyte resembles the acto-myosin dependent mechanism of localisation of Miranda during the asymmetric division of the neuroblasts [4], pointing to a more general role for Myo-II in the establishment of cellular asymmetry.
Questions to the authors:
- How is asymmetric Myosin-II distribution achieved? Is Myosin transported along microtubules in a kinesin-dependent manner? Is the EGF signalling modulating the activity of Unc-45 as well Myosin-II activation?
- Is there evidence of a difference lipid composition between the anterior and the posterior cortical domains? If yes, how do local changes in lipid composition affect establishment of cortical polarity?
References:
- Gonzalez-Reyes, A., H. Elliott, and D. St Johnston, Polarization of both major body axes in Drosophila by gurken-torpedo signalling. Nature, 1995. 375(6533): p. 654-8.
- Shulman, J.M., R. Benton, and D. St Johnston, The Drosophila homolog of C. elegans PAR-1 organizes the oocyte cytoskeleton and directs oskar mRNA localization to the posterior pole. Cell, 2000. 101(4): p. 377-88.
- Doerflinger, H., et al., The role of PAR-1 in regulating the polarised microtubule cytoskeleton in the Drosophila follicular epithelium. Development, 2003. 130(17): p. 3965-75.
- Hannaford, M.R., et al., aPKC-mediated displacement and actomyosin-mediated retention polarize Miranda in Drosophila neuroblasts. Elife, 2018. 7.
doi: https://doi.org/10.1242/prelights.29535
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the developmental biology category:
Dosage-sensitive RBFOX2 autoregulation promotes cardiomyocyte differentiation by maturing the transcriptome
Theodora Stougiannou
Post-translational Tuning of Human Cortical Progenitor Neuronal Output
Jawdat Sandakly
Aspartate transaminases are required for blood development
Hannah Pletcher
preLists in the developmental biology category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
BSDB/GenSoc Spring Meeting 2024
A list of preprints highlighted at the British Society for Developmental Biology and Genetics Society joint Spring meeting 2024 at Warwick, UK.
| List by | Joyce Yu, Katherine Brown |
GfE/ DSDB meeting 2024
This preList highlights the preprints discussed at the 2024 joint German and Dutch developmental biology societies meeting that took place in March 2024 in Osnabrück, Germany.
| List by | Joyce Yu |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
2nd Conference of the Visegrád Group Society for Developmental Biology
Preprints from the 2nd Conference of the Visegrád Group Society for Developmental Biology (2-5 September, 2021, Szeged, Hungary)
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Society for Developmental Biology 79th Annual Meeting
Preprints at SDB 2020
| List by | Irepan Salvador-Martinez, Martin Estermann |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EDBC Alicante 2019
Preprints presented at the European Developmental Biology Congress (EDBC) in Alicante, October 23-26 2019.
| List by | Sergio Menchero et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |











 (No Ratings Yet)
(No Ratings Yet)