Practical Fluorescence Reconstruction Microscopy for Large Samples and Low-Magnification Imaging
Posted on: 2 July 2020
Preprint posted on 12 May 2020
Categories: cell biology
Background
Deep learning holds great potential for biological microscopy data, and offers exciting opportunities for fluorescent feature reconstruction. Fluorescence reconstruction microscopy (FRM) takes in a transmitted light image of a biological sample and outputs a series of reconstructed fluorescence images that predict what the sample would look like had it been labeled with a given series of dyes or fluorescently tagged proteins. FRM works by first training a convolutional neural network to relate a large set of transmitted light data to corresponding real fluorescence images (the ground truth) for given markers. The network learns by comparing its fluorescence predictions to the ground truth fluorescence data and iterating until it reaches a cut off. Once trained, FRM can be performed on transmitted light data without requiring additional fluorescence imaging. Advantages to this include the possibility of reducing phototoxicity, freeing up fluorescence channels for more complex markers, and re-processing transmitted light data to extract new information. However, current FRM benchmarks are abstractions that are difficult to relate to how valuable or trustworthy an FRM prediction is. In their work, LeChance and Cohen aim to provide a standardized implementation of FRM, and demonstrate its practical performance and limitations for various conventionally performed cell biology analyses (1).
Key findings and developments
Development
U-Nets, and other deep learning approaches, have found broad applications to live-cell imaging tasks such as cell phenotype classification, feature segmentation, and histological stain analysis. The workflow presented in this work consists on collecting multi-channel training images of cultured cells whereby each image comprises a transmitted light channel and associated fluorescence channels. The images were broken into sub-images in ImageJ and input into the U-network for pattern recognition. The transmitted light images serve as input to the network and this process can be extended to full time-lapse microscopy fluorescence reconstruction. Pearson’s Correlation Coefficient (PCC) was selected as the conventional performance metric; however due to skewed results in images primarily with background, the authors report a corrected accuracy score (P) representing the PCC of a large subset of images in a given dataset containing positive examples of the feature of interest (eg nuclei) based on an intensity threshold. This approach aims to improve network performance for datasets containing large amounts of background signal.
Proof of concept
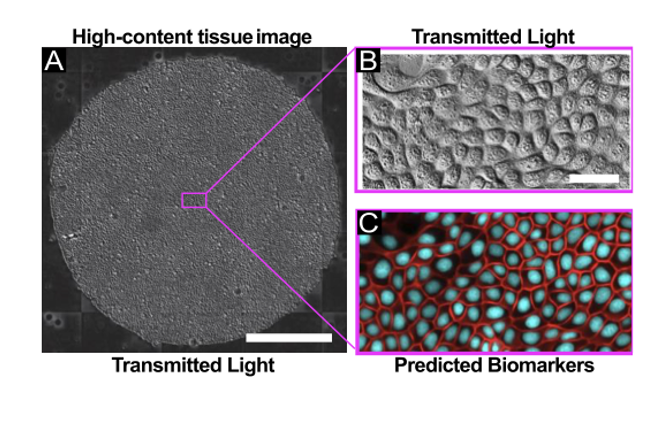
The authors explored FRM in the context of high-content imaging applications. For this, they captured transmitted light images using 4x, 10x, and 20x air objectives using either Phase Contrast or Differential Interference Contrast (DIC), and collected data across 3 different cell types. Moreover, the authors demonstrated the utility of low-magnification reconstruction and nuclear tracking in a 24h time-lapse of the growth dynamics of large epithelia, with images acquired every 10 minutes. FRM proved highly effective as an alternative to nuclear labeling approach for large-scale, long-term imaging, avoiding some shortcomings associated with the use of nuclear dyes.
The authors then went on to explore FRM in the context of reconstructing epithelial cell-cell junctions for segmentation and morphological studies. In the absence of specific markers, cell-cell junctions are relatively difficult to segment, especially from DIC images. In their work the authors show that the U-Net was able to reconstruct E-cadherin junctions with high visual accuracy, and determined that the FRM network is able to capture subtle 3D information from 2D input images.
The work then explores the possibility of finer reconstruction, by using a 20X/0.8NA objective, and HUVEC cells with multiple structures labeled. FRM had different levels of success for different structures, and the authors conclude that the value of FRM depends on the specific question and context. The conclude also that ultimately the decision on whether the detection of finer structures is good enough rests in the end user.
To facilitate information for such decision, the authors then provided several examples of how the size of the training affects the score P, and the accuracy of the resulting FRM predictions. In general, they show that FRM quality varies directly with the size of the training set, however, the rate of change in P vs. training set size is neither linear nor uniform across different biomarkers. They explored also the possibility of training a deeper network, but observed no significant improvement, leading to the conclusion that their minimal U-Net implementation performs well as a foundation for various types of analyses without the need for significant fine-tuning.
What I like about this preprint
I enjoyed this work a lot, and I think the tool the authors designed has a lot of potential for multiple fields.
Open questions
1.While in your work you aimed at proving the usefulness of the tool for high-throughput, how useful is its performance upon using a 63x or 100x objective? As you mention along your work, one of the advantages would be to overcome issues of photobleaching, and the possibility of freeing channels for visualizing other structures.
2.You mention the need of correction for PCC – (P), and that the skew you observe occurs in images with a lot of background. Can you expand further on this point?
3.Are there important artifacts you noticed, or further limitations to the use of your tool, beyond those you discuss?
4.You used various cell lines to test FRM. Following from the question above, are specific cell lines more complicated for FRM processing than others and if so, is it possible to generate training sets for specific cell lines a priori (ie already incorporated in the tool, and selectable by the user)?
5.Is your tool useful for more complex tissues such as histology sections?
References
- LeChance J and Cohen DJ, Practical fluorescence reconstruction microscopy for large samples and low-magnification imaging, bioRxiv, 2020.
doi: https://doi.org/10.1242/prelights.22546
Read preprintHave your say
Sign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
Kosmos: An AI Scientist for Autonomous Discovery
Roberto Amadio et al.
preLists in the cell biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
December in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cell cycle and division 2) cell migration and cytoskeleton 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Matthew Davies et al. |
November in preprints – the CellBio edition
This is the first community-driven preList! A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. Categories include: 1) cancer cell biology 2) cell cycle and division 3) cell migration and cytoskeleton 4) cell organelles and organisation 5) cell signalling and mechanosensing 6) genetics/gene expression
| List by | Felipe Del Valle Batalla et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |











 (No Ratings Yet)
(No Ratings Yet)
5 years
Julienne LaChance
Hi, this is Julie LaChance! Let me try to address your questions:
1.While in your work you aimed at proving the usefulness of the tool for high-throughput, how useful is its performance upon using a 63x or 100x objective? As you mention along your work, one of the advantages would be to overcome issues of photobleaching, and the possibility of freeing channels for visualizing other structures.
– Generally speaking, the higher the magnification, the better the prediction quality- you just have to make sure you have enough data! If you consider similar papers (one example: https://pubmed.ncbi.nlm.nih.gov/29656897/), you’ll see them tackling higher magnification images, where you can pick out fine details. In this paper we’re examining the low-mag story, to demonstrate how people can use these tools for large tissue analysis!
2.You mention the need of correction for PCC – (P), and that the skew you observe occurs in images with a lot of background. Can you expand further on this point?
– Yes! Since the PCC is a correlation coefficient, it’s a measure of how well the intensities in the ground truth image correlate with the intensities in the predicted image. So if you have two images with features in them (meaning: high intensities where there are features, and low intensities where it’s just background), and the prediction is working well, you’ll get a high PCC result. But, if the ground truth only contains background, and your predicted image is trying (and failing) to reproduce only background noise, you’re probably going to get a low PCC value. So for a fair comparison of feature reconstruction scores, we only consider images with features in them. Hope that makes sense!
3.Are there important artifacts you noticed, or further limitations to the use of your tool, beyond those you discuss?
– The usefulness of this tool is very much problem dependent! For example, if I want to get a sense for where nuclei are for tracking, this is likely a much easier problem than accurately reconstructing very fine features, like VE-cadherin fingers. Users should think carefully about downstream processing before applying the method, and whether they think there is rich enough information in the input images to produce the reconstructions they need. For example, very blurry imaging conditions probably won’t be useful for predicting fine structures.
I would love to see the field of FRM grow, to enable researchers to examine practical metrics for different applications of the method!
4.You used various cell lines to test FRM. Following from the question above, are specific cell lines more complicated for FRM processing than others and if so, is it possible to generate training sets for specific cell lines a priori (ie already incorporated in the tool, and selectable by the user)?
– Some cell lines are more complicated in the sense that they look “messier”: for example, our MDCK cells visually appear more uniform than our keratinocyte cells, which makes prediction slightly more challenging in the keratinocyte case. Nothing changes in the code, but you may need more data for messier cell lines, or may get slightly lower prediction scores, depending on the task.
And yes, you can absolutely generate trained model weights for different cell lines a priori. Our lab holds onto trained weights so that any time we need nuclei data (e.g. for density estimations or tracking), we can simply capture DIC or phase contrast timelapses and predict the nuclei later. Just make sure your imaging conditions are as close as possible to the conditions used in the training data!
5.Is your tool useful for more complex tissues such as histology sections?
– It could be! There exist a few other papers which do the same thing for histology: for example, https://www.nature.com/articles/s41377-019-0129-y .
Thanks for highlighting our work!