Astrocytes and neurons share brain region-specific transcriptional signatures
Posted on: 29 May 2020 , updated on: 8 April 2021
Preprint posted on 22 April 2020
Article now published in Science Advances at http://dx.doi.org/10.1126/sciadv.abe8978
Astrocytes can be reprogrammed to be neurons, but not any neuron! They show a preference for their “close neighbours” identity
Selected by Idoia Quintana-UrzainquiCategories: bioinformatics, developmental biology, neuroscience
Background
The brain holds an astonishing cell type diversity of both neurons and glia across different regions. Understanding this diversity and the reprograming potential from one cell type to the other will be instrumental for the development of regenerative therapies after brain injury. The brain regional diversity arises during embryonic development when progenitors from different regions activate specific transcriptional programs. We know that neurons born in the same area at a similar time tend to be similar to each other, at least at the level of gene expression. There is evidence that this might be also the case for other brain cell types like astrocytes1 . Given that neurons and glia often originate from the same progenitors2, do they share transcriptomic profiles? If so, is this similarity brain region-specific? In their last preprint, Herrero-Navarro et al. explore these questions by comparing astrocytes and neurons from developing cortex and thalamus in mice. The authors show that neurons and astrocytes share region-specific transcriptomic and epigenetic signatures and are clonally related. They also perform astrocyte-to-neuron reprogramming and find that their new identity matches that of their close “neuronal siblings”.
Key findings
Neurons and astrocytes share region-specific transcriptomic profiles
The authors first isolated astrocytes from different regions of P7 mice brains using a GFAP::GFP reporter mice line. They dissected the cortex (primary somatosensory region, S1) and three nuclei of the thalamus (dorsolateral geniculate dLG, ventral posteromedial VPM and ventro-medial geniculate MGv nuclei), processed the samples in a fluorescence-activated cell sorter, which isolated GFP-GFAP-positive astrocytes and performed bulk RNA-sequencing for each of the regions (Fig.1). Differential expression analysis between thalamic versus cortical samples showed that genes enriched in thalamic astrocytes are quite similar to genes normally expressed in thalamic neurons (Gbx2, Tcfl2, Rora, Lef1), while genes enriched in cortical astrocytes are reminiscent to those expressed in cortical neurons (Tbr1, Neurod6, Citip2, Satbt2). Additionally, they performed RNA-seq in thalamic neurons (Gbx2::tomato mouse line) from the same three nucleus and they analysed a published dataset of cortical neurons (Figure 1), confirming the high degree of similarity between top-enriched genes of neurons and astrocytes of the thalamus (37%) and between neurons and astrocytes from the cortex (17%). Analysis of a recent single cell dataset in juvenile mouse containing both neurons and astrocytes from the cortex and thalamus3 confirmed their findings. Interestingly, they also show that astrocytes and neurons from the three different thalamic nuclei cluster separately in PCA plots, meaning that their transcriptional signatures are also nucleus specific.
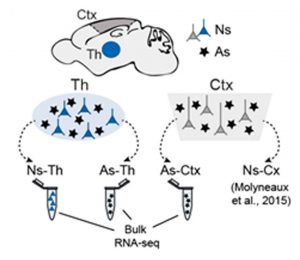
Thalamic neurons and astrocytes are clonally related and show little dispersion across boundaries
The authors next explore the clonal origin of neurons and astrocytes in the thalamus by performing a series of in utero electroporation with a battery of plasmids encoding different fluorofores under the control of GFAP promoter, following transposase mediated integration (“StarTrack”4 for the specific labelling of astrocytes) or a ubiquitous promoter. They electroporated at E11.5 and analysed the distribution of the clones at P8. They observed that both astrocyte-only clones and mixed clones (containing both astrocytes and neurons originated from the same progenitor) tend to remain in the boundaries of a given nucleus. This means that the similarity observed in the transcriptional signatures of astrocytes and neurons of the same regions can be explained by their shared clonal origin and their little dispersion throughout development. Moreover, these experiments also show that the positional identity of neurons and astrocytes is acquired at early developmental stages and retained postnatally.
Astrocytes can be reprogrammed to become neurons, but not any neuron, they specifically form neuronal types that closely resemble their anatomical neighbours. And this ability is cell-autonomous.
The authors next tested the ability of astrocytes to be reprogrammed to neurons following a previously published strategy that involves Ngn2-Bcl2-mediated neuronal induction5. Taking advantage that retroviruses only transduce proliferating glia at the stage they performed the experiment (P3), they first injected the retroviral vector in cortex and thalamus and found that newly reprogrammed neurons express their corresponding cortical or thalamic molecular markers (Fig. 2).
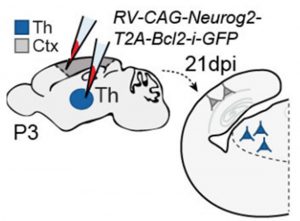
To discard the effect of the environment, they next used in vitro cultures of astrocytes from cortex and thalamus. They infected the astrocytes with a retrovirus carrying a Ngn2 expression construct, obtaining the same outcome. Cortical astrocytes become cortical neurons while thalamic astrocytes become thalamic neurons. To further control for the environment effect, they repeated these experiments but this time co-culturing the astrocytes with astrocytes or neurons from the other tissue, still observing the same outcome (Fig. 3).
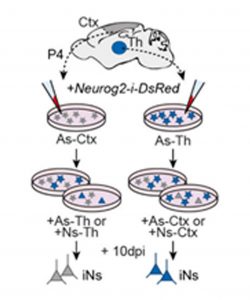
They even went further and tested this with astrocytes from different thalamic nuclei, finding that their reprogrammed neuronal identity reflects their nucleus of origin.
When trying to reprogram cortical astrocytes to thalamic neurons by using a thalamic fate determinant (Gbx2) in combination with Ngn2, they only caused a partial fate redirection, since only some thalamic signature genes were increased in these induced neurons.
These results indicate that, no matter what their environment is, astrocytes have an intrinsic tendency to form neurons of the same tissue. Finally, the authors find that epigenetic “primed” signatures inherited from progenitors could explain the differential induction potential of astrocytes born in different tissues, up to the level of different thalamic nuclei.
Why I find this preprint interesting
This preprint caught my eye because it deals with a very fundamental question in Developmental Biology (cell type specification, fate commitment and fate flexibility), and produces novel evidence with important biomedical implications. The authors’ assessment of fate flexibility of astrocytes is very elegant and proves they have the potential to become the neurons that surround them. This, I believe, makes them excellent targets for neuroregenerative therapies after brain injury.
Future directions and questions for the authors
- 1) In your clonal analysis using the ubiquitous promoter you find that mixed clones (giving rise to both neurons and astrocytes) tend to remain within a given thalamic nucleus territory while “only-neuron” _clones spread more broadly. Do you have any hypothesis to explain this different behaviour? Who might be these neurons that expand more broadly? Are they related with any known tangential migration?
This is a very good question. Our data when looking only at the neurons reveals a slightly higher dispersion than in astrocytes. But it is important to mention that the number of clones containing only neurons is quite low. This result is, in any case, in agreement with previous studies (see Song-Hai Shi or Nakagawa’s work). Thalamic astrocytes seem to have a restricted allocation preference to a single nucleus, and we hypothesize that this might be mainly due to the timing of generation, and the position of the progenitor at the time of astrogenesis.
2) Your study and others4 demonstrate that astrocyte reprogramming potential seems to be tissue-, cortical layer- and even thalamic nucleus- specific. This reinforce the idea of astrocytes as powerful candidates for local brain repair after injury (versus their classic view as the harmful cell type which form the glial scar) or in disease. Where is this field now? Where do you see it going next?
We consider that this topic very promising and with lot of potential. Several groups have also made interesting discoveries that confirm that astrocytes are good candidate cells for brain repair, and our data is push even more into this direction by showing that astrocytes are molecularly “primed’’ to a region. Astrocytes have also the advantage to be resident cells that will not induce any immune response as it could occur with exogenous cell transplantation. Currently we consider that the field is moving towards finding less invasive reprogramming in vivo. The AAV delivery seems to be quite promising, and it is becoming the preferred option for targeting astrocytes in vivo. Finally, the next step of course will be to confirm the complete recovery of a long-range connection, which in fact has been recently shown in a few papers, and to confirm a functional recovery of a damaged circuit after reprogramming.
3) Astrocytes do not spontaneously reprogram into neurons after brain damage in mammals (at least in the species that have been studied). But they do seem to hold the potential to do so. Do astroglial counterparts in more ancient animal groups have this ability? In other words, is this potentiality a remnant of an ability that was lost in modern vertebrates?
Well, we are not experts in the properties or functions of astrocytes from an evolutionary point of view, but this is an interesting question. It is true that the nervous system in simpler animal models seems to be more plastic and with a higher capacity for neuronal regeneration, which is lost in superior vertebrates. We are not aware that astroglial cells could spontaneously be converted into neurons in response to damage. Perhaps, in more ancient animal, astrocytes cells remain in a more immature or less specialized state, making them easier to change their identity even spontaneously after a damage.
References
- Hochstim, C., Deneen, B., Lukaszewicz, A., Zhou, Q. & Anderson, D. J. (2008). Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell 133, 510–522
- Rowitch, D. H. & Kriegstein, A. R. Developmental genetics of vertebrate glial cell specification (2010). Nature 468, 214–222.
- Zeisel A, Hochgerner H, Lönnerberg P, et al. (2018). Molecular Architecture of the Mouse Nervous System. Cell 174, 999-1014.
- García-Marqués, J. & López-Mascaraque, L. (2013) Clonal identity determines astrocyte cortical heterogeneity. Cortex 23, 1463–1472.
- Gascón S, Murenu E, Masserdotti G, et al. (2016). Identification and Successful Negotiation of a Metabolic Checkpoint in Direct Neuronal Reprogramming. Cell Stem Cell 18, 396–409.
- Mattugini N, Bocchi R, Scheuss V, et al. (2019) Inducing Different Neuronal Subtypes from Astrocytes in the Injured Mouse Cerebral Cortex. Neuron 103, 1086–1095.
doi: https://doi.org/10.1242/prelights.21264
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the bioinformatics category:
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Kosmos: An AI Scientist for Autonomous Discovery
Roberto Amadio et al.
Human single-cell atlas analysis reveals heterogeneous endothelial signaling
Charis Qi
Also in the developmental biology category:
Cooperation between cortical and cytoplasmic forces shapes planar 4-cell stage embryos
Corentin Mollier, Shivani Dharmadhikari
A drought stress-induced MYB transcription factor regulates pavement cell shape in leaves of European aspen (Populus tremula)
Jeny Jose
Cross Sectional and Longitudinal Imaging Reveals Spatiotemporal Divergence in Morphogenesis and Cell Lineage Specification between in-vivo and in-vitro Mouse Embryo during Pre- and Peri-implantation
Heather Pollington
Also in the neuroscience category:
Electrophysiological correlates of conscious experiences during sleep: Lucid dreams, sleep paralysis, out-of-body experiences, and false awakenings
uMontreal Neuro preLighters et al.
PPARδ activation in microglia drives a transcriptional response that primes phagocytic function while countering inflammatory activation
Isabel Paine
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
preLists in the bioinformatics category:
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Also in the developmental biology category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
BSDB/GenSoc Spring Meeting 2024
A list of preprints highlighted at the British Society for Developmental Biology and Genetics Society joint Spring meeting 2024 at Warwick, UK.
| List by | Joyce Yu, Katherine Brown |
GfE/ DSDB meeting 2024
This preList highlights the preprints discussed at the 2024 joint German and Dutch developmental biology societies meeting that took place in March 2024 in Osnabrück, Germany.
| List by | Joyce Yu |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
2nd Conference of the Visegrád Group Society for Developmental Biology
Preprints from the 2nd Conference of the Visegrád Group Society for Developmental Biology (2-5 September, 2021, Szeged, Hungary)
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Society for Developmental Biology 79th Annual Meeting
Preprints at SDB 2020
| List by | Irepan Salvador-Martinez, Martin Estermann |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EDBC Alicante 2019
Preprints presented at the European Developmental Biology Congress (EDBC) in Alicante, October 23-26 2019.
| List by | Sergio Menchero et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |
Also in the neuroscience category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |











 (2 votes)
(2 votes)