Hedgehog signaling is required for endomesodermal patterning and germ cell development in Nematostella vectensis
Posted on: 25 February 2020
Preprint posted on 15 January 2020
Article now published in eLife at http://dx.doi.org/10.7554/elife.54573
On the (h)edge: the germline precursors of a basal metazoa are induced at the interface between Hedgehog signalling domains
Selected by Paul Gerald L. Sanchez and Stefano VianelloCategories: developmental biology, evolutionary biology
Background
Organisms that reproduce sexually do so by fusion of two specialised cells: sperm and egg (gametes). By carrying genetic information from one generation to the other these cells ensure the maintenance of the species over time. While in some organisms (e.g. plants) gametes are derived from cells that also give rise to body types, in (most) animals this is not the case. Sperm and eggs are instead produced by a dedicated line of cells put aside very early as to spare them from the accumulation of mutations over time. Throughout reproductive life gametes will be produced by dedicated, germline, stem cells. In turn, these cells have their origin in the embryo and ultimately descend from so-called Primordial Germ Cells (PGCs).
As the early setting aside of PGCs will ultimately originate the germline, PGC specification is a milestone of early development, and understanding the mechanisms leading to their specification is an area of great interest. Comprehensive surveys of PGC specification across the evolutionary tree report two main strategies through which embryos specify their PGCs:
- preformation, whereby maternal factors are quickly deposited within a subset of cells in the embryo to lock them into PGC fate
- induction, whereby PGCs are specified later by signalling cues from the zygote/embryo itself (e.g. BMP signalling in mouse)
Most model organisms such as C.elegans (roundworm), Drosophila (fruitfly), zebrafish, and Xenopus (frog) all specify their PGCs by preformation, while induction seems to be an exclusive of e.g. chicken, mouse, and human. While preformation might thus intuitively appear to be “ancestral”, most species actually specify PGCs by inductive mechanisms. Even more interestingly, research on cnidarians (sister group of all bilaterians) has found that here too PGCs are induced rather than locked away by early maternal determinants. The common ancestor of all animals would thus induce their PGCs, but how this actually takes place has remained a mystery.
Key Findings
The authors identify the mechanisms of PGC specification in the sea anemone Nematostella vectensis (bilaterian sister group), and confirm that here too specification occurs by induction rather than preformation. Specifically, PGCs are induced out of a field of endomesodermal cells (which surround the pharynx), where these cells abut the ectoderm of the pharynx itself. As can be seen in Figure A, this interface and site of PGC specification crucially coincides with the interface between Hedgehog-signalling domains (Hh and its receptor, Patched).
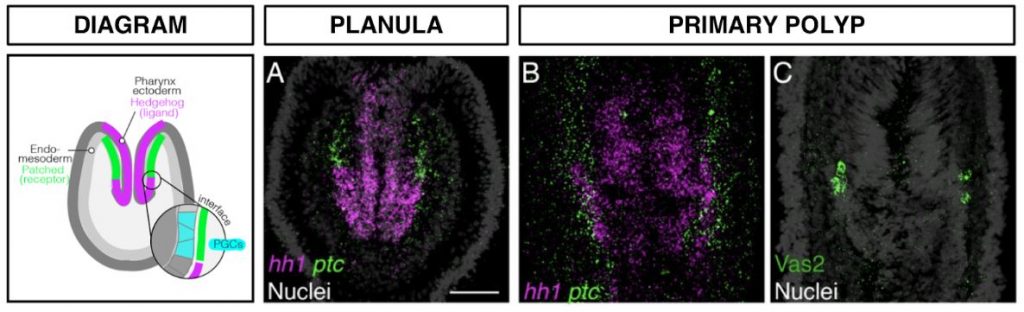
FIGURE A.
Hedgehog-signaling expression domains, showing juxtaposed expression of the ligand (hh1) and its receptor (ptc). Putative PGC clusters, marked by the Vasa2 protein, form at the interface of the two expression domains. [Modified from Figure 5 of the preprint. Red was changed to magenta using the “Replace Red with Magenta” plug-in in Fiji.]
While maternally inherited germline-determinants (e.g. Vasa2) are indeed detected, these do not differentially accumulate in any cell, and germline transcripts are expressed homogeneously throughout the entire endomesodermal field. Instead, one observes clear segregation between cells expressing the signalling molecule Hedgehog (the pharynx ectoderm), and cells expressing its receptor Patched (the surrounding endomesoderm). At the interface between these domains, where Hedgehog signalling is thus productive, PGCs are specified.
The authors then track these cells as they delaminate out of the endomesoderm by epithelial to mesenchymal transition (EMT)-like mechanisms, and reach the developing gonad rudiments, in which they will mature to germline stem cells.
Results are particularly interesting in the use of Hox mutants, altered in their body plan, where PGCs specify in altered locations (Figure B), indicative of an inductive mode of specification. That is, even though these mutants miss the primary structures where PGCs would be expected to emerge (primary mesenteries), PGCs emerge nonetheless, if in an altered location. Conversely, even in the wild-type body plan, PGCs will not get specified if Hedgehog signalling is absent (CRISPR experiments).
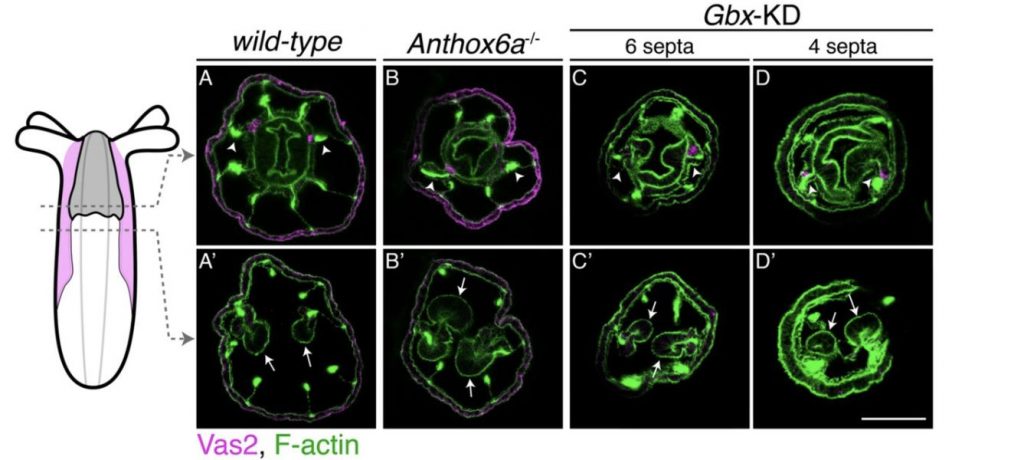
FIGURE B.
Localization of putative PGC clusters in Hox mutants with altered body plans. [Figure S7 of the preprint. Red was changed to magenta using the “Replace Red with Magenta” plug-in in Fiji.]
Significance
The current study builds on the beautiful body of previous work that has investigated the formation of precursor germ cells in a basal metazoa, Nematostella vectensis. The authors provide here several pieces of evidence supporting inductive PGC specification (versus preformation) as an ancestral strategy in eumetazoans, and they capitalize on experimental advances that were not previously available (e.g. immunostaining with antibody against Vasa2 and generation of mutants using CRISPR/Cas9).
The identification of the role of Hedgehog signaling in specification of PGCs is remarkable and sparks interesting developmental questions. Most noteworthy is the description of the reciprocal expression of the Hedgehog ligand and its receptor, and how putative PGCs (Vasa2+ cells) form at the interface of these juxtaposed expression domains.
The preprint provides thought-provoking mechanistic insight into the possible earliest forms of animal PGC specification.
Questions to the authors:
- What is known about the role of Hedgehog signalling for PGC induction in other organisms and model systems? Are there traces (or conservation) of such ancestral evolutionary strategy?
- Would it be possible to implant a bead with Hh ligand (hh1) in the endomesoderm of the planula, adjacent to the domain expressing the Hh receptor (ptc)? Would this result in specification of an ectopic putative PGC cluster?
- BMP signalling is described here as having a purely attractive/repulsive role, almost like a chemokine. This is in contrast with its key PGC-inductive role usually associated to it based on studies in e.g. mouse (and even cricket, cfr. Extavour and Nakamura 2016). Could you elaborate on this?
- It is particularly remarkable that PGCs form near the mouth in Nematostella, while their site of induction is posterior in mouse (and human?). Could a model be made whereby the site of PGC specification correlates with the site of Gastrulation?
- If zygotic specification is ancestral, what would be the model explaining loss of this strategy in favour of determinant deposition (preformation) in some later species?
Further Reading
- Extavour, Cassandra G., and Michael Akam. “Mechanisms of germ cell specification across the metazoans: epigenesis and preformation.” Development 130.24 (2003): 5869-5884.
- Extavour, Cassandra G., et al. “Vasa and nanos expression patterns in a sea anemone and the evolution of bilaterian germ cell specification mechanisms.” Evolution & development 7.3 (2005): 201-215.
- Johnson, Andrew D., and Ramiro Alberio. “Primordial germ cells: the first cell lineage or the last cells standing?.” Development 142.16 (2015): 2730-2739.
- He, Shuonan, et al. “An axial Hox code controls tissue segmentation and body patterning in Nematostella vectensis.” Science 361.6409 (2018): 1377-1380.
- Matus, David Q., et al. “The Hedgehog gene family of the cnidarian, Nematostella vectensis, and implications for understanding metazoan Hedgehog pathway evolution.” Developmental biology 313.2 (2008): 501-518.
doi: https://doi.org/10.1242/prelights.16775
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the developmental biology category:
A drought stress-induced MYB transcription factor regulates pavement cell shape in leaves of European aspen (Populus tremula)
Jeny Jose
Cross Sectional and Longitudinal Imaging Reveals Spatiotemporal Divergence in Morphogenesis and Cell Lineage Specification between in-vivo and in-vitro Mouse Embryo during Pre- and Peri-implantation
Heather Pollington
Tissue mechanics and systemic signaling safeguard epithelial tissue against spindle misorientation
Ruoheng Li
Also in the evolutionary biology category:
A drought stress-induced MYB transcription factor regulates pavement cell shape in leaves of European aspen (Populus tremula)
Jeny Jose
Morphological variations in external genitalia do not explain the interspecific reproductive isolation in Nasonia species complex (Hymenoptera: Pteromalidae)
Stefan Friedrich Wirth
A high-coverage genome from a 200,000-year-old Denisovan
AND
A global map for introgressed structural variation and selection in humans
Siddharth Singh
preLists in the developmental biology category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
BSDB/GenSoc Spring Meeting 2024
A list of preprints highlighted at the British Society for Developmental Biology and Genetics Society joint Spring meeting 2024 at Warwick, UK.
| List by | Joyce Yu, Katherine Brown |
GfE/ DSDB meeting 2024
This preList highlights the preprints discussed at the 2024 joint German and Dutch developmental biology societies meeting that took place in March 2024 in Osnabrück, Germany.
| List by | Joyce Yu |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
2nd Conference of the Visegrád Group Society for Developmental Biology
Preprints from the 2nd Conference of the Visegrád Group Society for Developmental Biology (2-5 September, 2021, Szeged, Hungary)
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Society for Developmental Biology 79th Annual Meeting
Preprints at SDB 2020
| List by | Irepan Salvador-Martinez, Martin Estermann |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EDBC Alicante 2019
Preprints presented at the European Developmental Biology Congress (EDBC) in Alicante, October 23-26 2019.
| List by | Sergio Menchero et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |
Also in the evolutionary biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
EMBO | EMBL Symposium: The organism and its environment
This preList contains preprints discussed during the 'EMBO | EMBL Symposium: The organism and its environment', organised at EMBL Heidelberg, Germany (May 2023).
| List by | Girish Kale |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
COVID-19 / SARS-CoV-2 preprints
List of important preprints dealing with the ongoing coronavirus outbreak. See http://covidpreprints.com for additional resources and timeline, and https://connect.biorxiv.org/relate/content/181 for full list of bioRxiv and medRxiv preprints on this topic
| List by | Dey Lab, Zhang-He Goh |
1
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |











 (1 votes)
(1 votes)