Selective inhibitors of JAK1 targeting a subtype-restricted allosteric cysteine
Posted on: 13 March 2022
Preprint posted on 1 February 2022
JAK of 1 trade: researchers discover an inhibitor selective for a Janus tyrosine kinase subtype-specific cysteine residue
Selected by Zhang-He GohCategories: bioengineering, pharmacology and toxicology, synthetic biology
Background of the preprint
Janus tyrosine kinases (JAKs) are important proteins responsible for regulating cytokine signalling in the body and comprise 4 subtypes (JAK1, JAK2, JAK3, and TYK2). JAKs have been implicated in multiple diseases, ranging from immune conditions to cancer, making them attractive drug targets. However, current JAK inhibitors are not currently highly selective for specific JAK isoforms. Considering the multiple physiological roles and widespread distribution of JAKs throughout the body, available therapeutic JAK inhibitors to date have many side effects arising from their lack of specificity.
To that end, there is a need for specific JAK inhibitors that can discriminate between the various JAK isoforms. In this work, Kavanagh et al. target this challenge, applying electrophilic fragments to proteomic studies (Fig. 1). This strategy led them to discover a ligandable allosteric cysteine in JAK1 (C817) and TYK2 (C838) which is not present in JAK2 and JAK3. Through subsequent optimisation, the authors generated the electrophilic compound VVD-118313, an inhibitor selective for JAK1 and TYK2 over JAK2 and JAK3. VVD‑118313 specifically inhibits cytokine pathways that rely on JAK1’s phosphorylation activity and scaffolding functions while sparing cytokine pathways relying on other JAK subtypes.
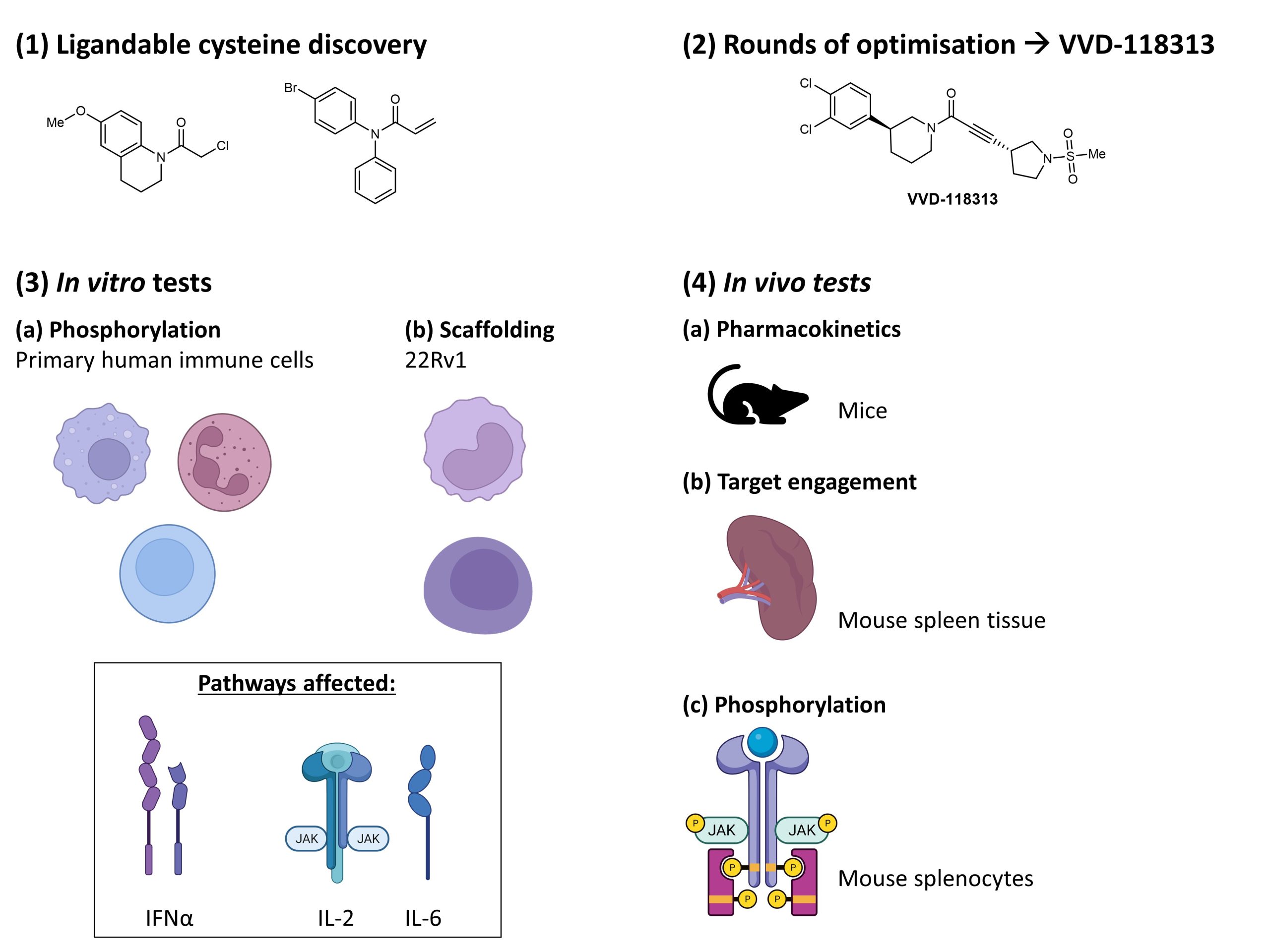
Figure 1. An overview of work described in the preprint by Kavanagh et al..
Key findings of this preprint
First, Kavanagh et al. determined that the ligandable cysteine, which was discovered in previous activity-based protein profiling (ABPP) studies, could react with chloroacetamide and acrylamide fragments. The authors then performed a subsequent screen of this ligandable cysteine against a library of electrophilic compounds, identifying a fragment hit. Further rounds of optimisation led them to identify VVD-118313 as the best performing compound.
Next, the authors evaluated the reactivity of VVD-118313. Their experiments identified that C817 in JAK1 and C838 in TYK2 were most affected by VVD-118313, confirming the selectivity and potency of VVD-118313. By evaluating the ability of VVD‑118313 to perturb JAK1 and TYK2 signalling pathways, the authors found that VVD‑118313 was selective for JAK1 over JAK2 (unlike the JAK inhibitor tofactinib which inhibited both JAK1 and JAK2) and confirmed that VVD‑118313 suppressed TYK2 signalling. These experiments also confirmed that the C817 residue on JAK1 and C838 in TYK2 are crucial for VVD‑118313 activity.
In vitro tests on primary human immune cells showed that both VVD-118313 and its mixture of stereoisomers inhibited various pathways involving interferons (IFNα) and interleukins (IL‑6 and IL‑2), with VVD‑118313 being the more potent inhibitor. Kavanagh et al. also performed in vivo tests using the mixture of stereoisomers. Despite the stereoisomeric mixture’s suboptimal pharmacokinetic properties—a short half-life (t1/2 = 0.36 h) and rapid clearance in mice (CL = 112 ml/min/kg)—the authors reasoned that the covalent mechanism of action of the stereoisomeric mixture would still enable it to be effective in vivo. Indeed, they observed 75% engagement of C816 in mouse spleen tissue and substantial impairments in IFNα‑dependent STAT1 phosphorylation in mouse splenocytes. These findings confirmed that the covalent ligands, as represented by the stereoisomeric mixture of VVD‑118313, selectively disrupt JAK1-dependent cytokine signalling in human and mouse immune cells.
Finally, Kavanagh et al. explored the activity of VVD‑118313 on the scaffolding roles of JAK1 and JAK2. In experiments involving the human prostate carcinoma cell line 22Rv1, VVD‑118313 did not affect cells in which C817 was substituted, thus confirming that VVD‑118313 operates through C817 in JAK1. VVD‑118313 inhibited IFNα- and IFNγ‑mediated phosphorylation of STAT1 but not GM‑CSF‑mediated phosphorylation of STAT5 in primary human immune cells. Further, VVD‑118313 also inhibited JAK1‑dependent IFNα‑STAT1, IL‑6‑STAT3 signalling, and, partially, IL‑2‑STAT5 signalling in human peripheral blood mononuclear cells (PBMCs). In these experiments, the inhibitory profile of VVD‑118313 differed significantly from other tested JAK inhibitors (tofacitnib, upadacitinib, itacitinib) and TYK2 inhibitor BMS‑986165.
What I like about this preprint
I selected this preprint to highlight the broad applicability of chemical biology to drug discovery. Scientific advances in the last five decades have enabled researchers to overcome past challenges traditionally associated with covalent inhibitors, leading to growing scientific output.1 This has yielded remarkable results: the strategy of covalent inhibition has been used to develop antivirals against SARS-CoV-2, the virus responsible for the COVID-19 pandemic outbreak.2
Importantly, this preprint by Kavanagh et al. demonstrates the close ties between proteomic research and drug discovery. In utilising a fragment approach, the authors show how the chemical methods that were widely developed for protein labelling can be harnessed to identify potential targets for drug discovery. It is particularly impressive that the authors carried the discovery of VVD‑118313 through to in vivo experiments and even characterised its pharmacokinetics.
Future directions
Kavanagh et al. have highlighted several main areas in moving forward: (1) improvements in the pharmacokinetics of VVD‑118313 before performing further in vivo studies, and (2) exploration of the structure-activity relationship of VVD-118313 to optimise for greater potency and deliver dual allosteric inhibitors for JAK1 and TYK2. In addition to these two points, the authors have also alluded to the exciting possibility that these specific JAK1 inhibitors may help to reduce the systemic toxicities associated with non-specific JAK inhibition.
Given the close relationship between chemical proteomics and drug discovery, another area that could be explored would be to employ high-throughput experimentation to expedite the discovery process. The key advantage here lies in speed—screening compounds using automation would allow researchers to perform thousands of small-scale experiments in a day, saving them time and effort. Cost savings may also be gained from such a platform: not only are proteins are expensive to acquire, but the synthesis of highly reactive, enantiopure probes is also a non-trivial challenge. In this preprint, the authors obtained enantiopure VVD‑118313 by employing chromatography to achieve chiral separation, a strategy that sufficed for early studies but proved to be a more significant challenge on a larger scale for subsequent in vivo studies.
Therefore, I think the chemical biology community would also benefit greatly from newer methods for more efficient chemical synthesis. Better enantioselective reactions will help researchers working at the chemistry-biology interface to generate the compounds that they need to perform biological assays. This would, in turn, yield better insights into the cellular mechanisms that are being probed.
Questions for authors
- Given that the 22Rv1 cell line is a human prostate epithelial cell line, why were 22Rv1 cells used in this study? Was it due to the ease of transfection, or were there other considerations?
- The labelling reaction between C817 and VVD‑118313 is very fast (the reaction is completed within 1-2 h), effective, and selective. What could be the cause for this selectivity and rapid reaction? For instance, is C817 particularly reactive compared to other cysteines?
References
- Sutanto, F.; Konstantinidou, M.; Dömling, A., Covalent inhibitors: a rational approach to drug discovery. RSC Med. Chem. 2020, 11 (8).
- Cully, M., A tale of two antiviral targets-and the COVID-19 drugs that bind them. Nature reviews. Drug discovery 2021.
doi: https://doi.org/10.1242/prelights.31622
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the bioengineering category:
A Novel Chimeric Antigen Receptor (CAR) - Strategy to Target EGFRVIII-Mutated Glioblastoma Cells via Macrophages
Dina Kabbara
Human pluripotent stem cell-derived macrophages modify development of human kidney organoids
Theodora Stougiannou
Matrix viscoelasticity regulates dendritic cell migration and immune priming
Roberto Amadio
Also in the pharmacology and toxicology category:
Snake venom metalloproteinases are predominantly responsible for the cytotoxic effects of certain African viper venoms
Daniel Osorno Valencia
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
In vitro pharmacokinetics and pharmacodynamics of the diarylquinoline TBAJ-587 and its metabolites against Mycobacterium tuberculosis
Zhang-He Goh
Also in the synthetic biology category:
Enzymatic bromination of native peptides for late-stage structural diversification via Suzuki-Miyaura coupling
Zhang-He Goh
Enhancer cooperativity can compensate for loss of activity over large genomic distances
Milan Antonovic
Discovery and Validation of Context-Dependent Synthetic Mammalian Promoters
Jessica L. Teo
preLists in the bioengineering category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Advances in microscopy
This preList highlights exciting unpublished preprint articles describing advances in microscopy with a focus on light-sheet microscopy.
| List by | Stephan Daetwyler |
Also in the pharmacology and toxicology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
COVID-19 / SARS-CoV-2 preprints
List of important preprints dealing with the ongoing coronavirus outbreak. See http://covidpreprints.com for additional resources and timeline, and https://connect.biorxiv.org/relate/content/181 for full list of bioRxiv and medRxiv preprints on this topic
| List by | Dey Lab, Zhang-He Goh |
1
Drug use in special populations
Any drugs that are being used in special populations: Patients with liver and kidney failure, in paediatrics, in geriatrics, and in pregnant or lactating patients. Includes the discovery of factors that could potentially affect drug use in these special populations.
| List by | Zhang-He Goh |
Toxicology of toxicants, existing therapeutics, and investigational drugs
Preprints that describe the toxicology of environmental pollutants and existing and upcoming drugs. Includes both toxicokinetics and toxicodynamics, as well as technological improvements that will help in the characterisation of this field.
| List by | Zhang-He Goh |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Anticancer agents: Discovery and clinical use
Preprints that describe the discovery of anticancer agents and their clinical use. Includes both small molecules and macromolecules like biologics.
| List by | Zhang-He Goh |
Advances in Drug Delivery
Advances in formulation technology or targeted delivery methods that describe or develop the distribution of small molecules or large macromolecules to specific parts of the body.
| List by | Zhang-He Goh |
Also in the synthetic biology category:
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)