SOL1 and SOL2 Regulate Fate Transition and Cell Divisions in the Arabidopsis Stomatal Lineage
Posted on: 3 September 2018
Preprint posted on 17 August 2018
Article now published in Development at http://dx.doi.org/10.1242/dev.171066
Changing a cell’s destiny: SOL1 and SOL2 are novel regulators of cell fate in the Arabidopsis stomatal lineage
Selected by Martin BalcerowiczCategories: developmental biology, plant biology
Background: Arabidopsis stomatal development is controlled by the sequential action of three bHLH transcription factors
Stomata are epidermal pores that enable controlled gas exchange across the leaf’s surface while restricting water loss and are thus crucial for a plant’s survival. In Arabidopsis, they are formed by a distinctive set of cell divisions and cell state transitions. Protodermal cells enter the stomatal cell lineage through an asymmetric entry division that gives rise to a smaller cell, the meristemoid, and a larger stomatal lineage ground cell (SLGC). The meristemoid has limited self-renewing capability, it can undergo further asymmetric amplifying divisions and thereby produce additional SLGCs. Eventually, the meristemoid then differentiates into a guard mother cell (GMC), which divides once symmetrically to give rise to the two guard cells (GCs) that form a stoma (Fig. 1). The surrounding SLGCs can either differentiate into pavement cells – the puzzle-shaped basic cell type of the epidermis – or can themselves undergo another asymmetric division, giving rise to a satellite meristemoid. This division is referred to as a spacing division as the new meristemoid is formed away from the existing meristemoid/stoma, ensuring that two stomata are always separated by at least one non-stomatal cell (“one cell spacing rule”).
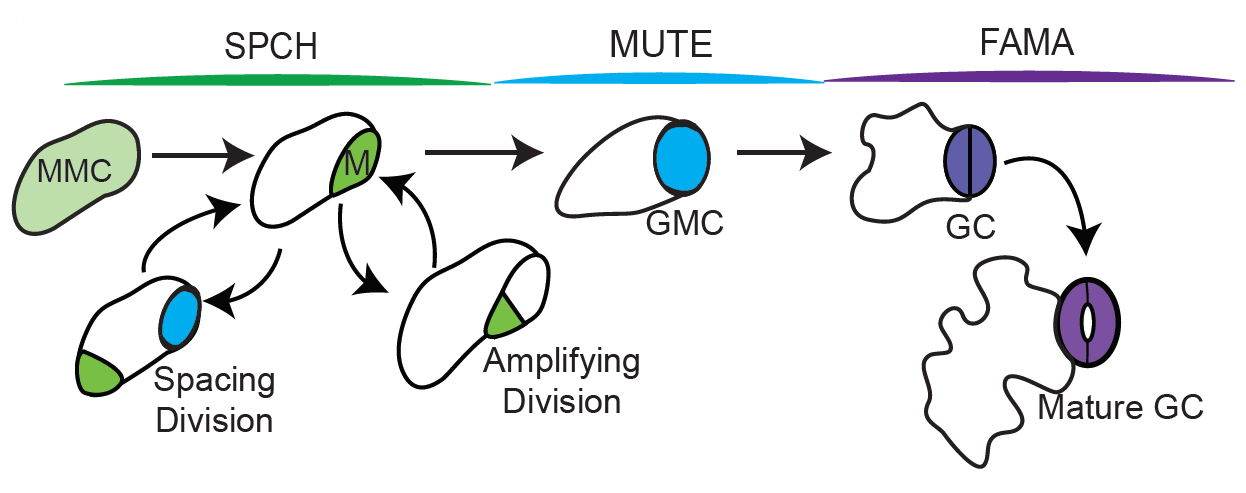
Cell divisions and cell state transitions in the stomatal lineage are controlled by the sequential action of three closely related bHLH transcription factors1, 2: SPEECHLESS (SPCH) promotes entry, amplifying and spacing divisions; MUTE represses meristemoid identity in the GMC and triggers its symmetric division to form GCs; eventually, FAMA prevents further division of the GCs and locks them in a differentiated state. How these processes are controlled downstream of these transcription factors has only begun to be understood. In their preprint, Simmons, Davies et al. report the cysteine-rich repeat (CXC)-hinge-CXC (CHC) proteins TSO-LIKE1 (SOL1) and SOL2 as novel SPCH targets, whose function is required for several cell state transitions throughout the stomatal lineage.
Key findings: SOL1 and SOL2 are required for multiple cell state transitions in the stomatal lineage
Genes directly bound and upregulated by SPCH had been identified in a previous study by the Bergmann lab3. Two of these direct targets – SOL1 and SOL2 – are homologues of animal DNA-binding components of DREAM complexes, protein complexes involved in cell cycle regulation and cell differentiation4. As the SOLs’ function in Arabidopsis was unknown, the authors investigated a potential function of SOL1 and SOL2 in stomatal development.
Expression of SOL transcriptional and translational reporters was detected in meristemoids and GMCs, following the expression of SPCH-CFP and MUTE-CFP reporters, respectively. Time lapse imaging further pinned down the precise timing of SOL protein accumulation – their signals disappeared just before the division of the meristemoid or GMC, suggesting that their accumulation is tightly linked to the cell cycle.
To further understand SOL function, the authors went on to characterise T-DNA insertion mutants. While single sol1 and sol2 mutants showed no apparent defect in epidermal patterning, a sol1 sol2 double mutant displayed an increased number of small meristemoid-like cells occurring in clusters in a young leaf’s epidermis and pairs of stomata in direct contact in the epidermis of mature leaves. Time lapse imaging revealed that these pairs can form in two different ways: (1) Two meristemoids in a cluster of four may differentiate into GMCs, with the subsequent symmetrical divisions giving rise to two stomata in contact; (2) GCs may divide symmetrically a second time to form four GCs. Thus, SOLs appear to have multiple roles: as downstream targets of SPCH they promote the transition of meristemoids into GMCs, but they are also required at the following stage (possibly downstream of MUTE), restricting the GMC to a single symmetric division (Fig. 2).
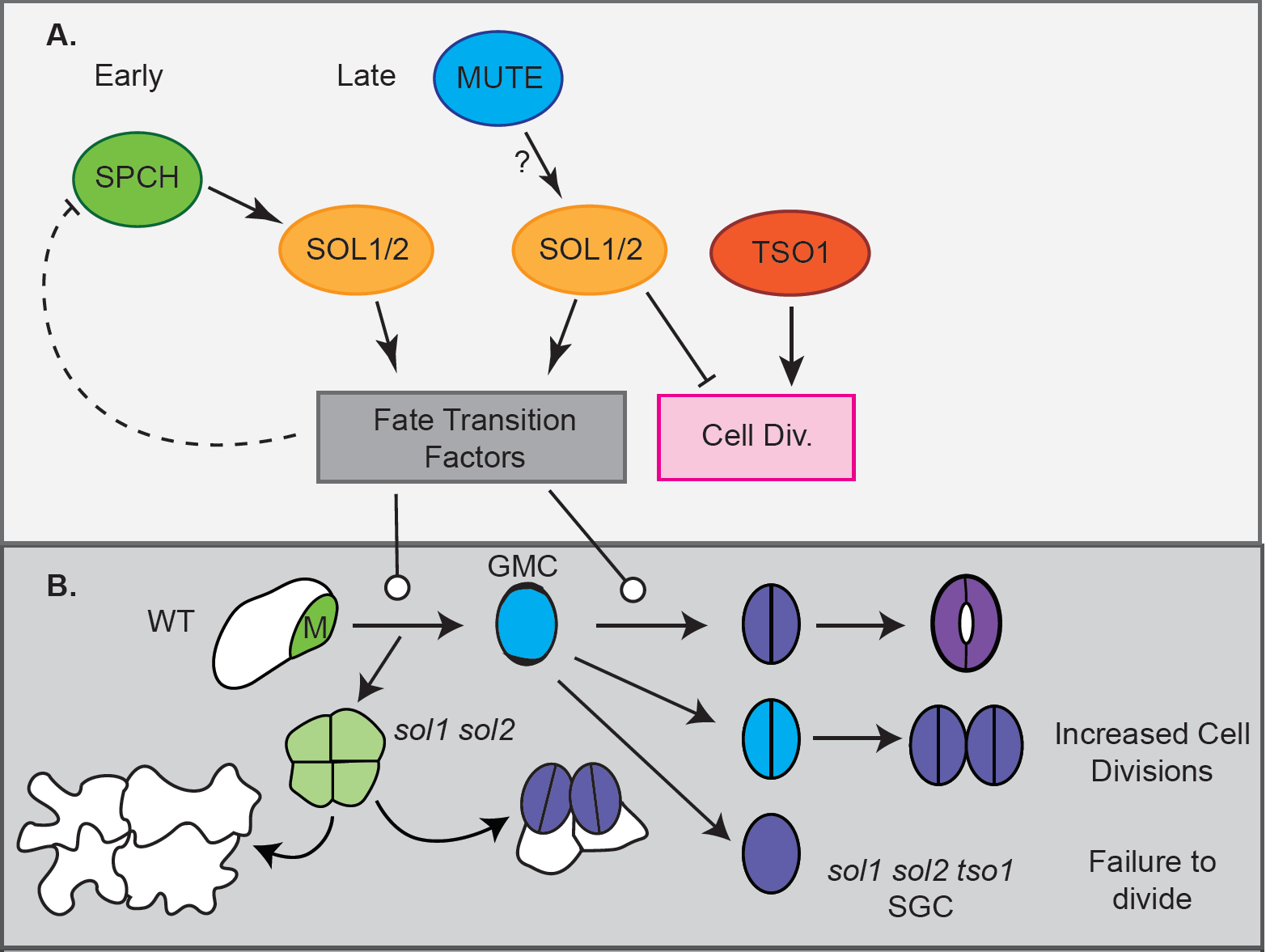
The closest paralogue of SOL1 and SOL2, TSO1, also turned out to be involved in stomatal development. Whereas tso1 single mutants did not display altered epidermal patterning, knock-down of TSO1 in the sol1 sol2 background led to the formation of single guard cells (SGCs) that failed to divide, a phenotype also generated by SOL2 overexpression. Thus, TSO1 appears to act partially in opposition to SOL1 and SOL2, promoting the final GMC division.
What I like about this preprint
How patterns emerge during tissue and organ formation is a fundamental question in developmental biology. The present study does a remarkable job at shining more light onto one of these processes: it identifies novel regulators of stomatal patterning downstream of known master regulators, gives us an idea about their function in early and late stages of the process and opens up several lines of research that can be followed from here on.
Open questions/future research: DREAMing of CHC protein function in Arabidopsis
With novel functions assigned to some Arabidopsis CHC proteins, this protein family may draw more interest in the future. Some open questions that may be addressed are:
- There are eight CHC proteins in Arabidopsis, five of them uncharacterised – do they they have a role in stomatal development as well?
- Animal CHC proteins are part of DREAM multiprotein complexes – is this also the case for the SOLs in plants?
- SOLs may function as repressors of the cell cycle – which targets do they control to achieve this function?
- Lack of SOLs slows the transition from meristemoid to GMC, but does not prevent it completely. Which proteins contribute to this transition alongside the SOLs?
References/Further reading
- Han, SK, and Torii, KU (2016). Lineage-specific stem cells, signals and asymmetries during stomatal development. Development 143(8): 1259-70.
- Lau, OS, and Bergmann, DC (2012). Stomatal development: a plant’s perspective on cell polarity, cell fate transitions and intercellular communication. Development 139(20): 3683–3692
- Lau, OS, Davies, KA, Chang, J, Adrian, J, Rowe, MH, Ballenger, CE, Bergmann, DC (2014). Direct roles of SPEECHLESS in the specification of stomatal self-renewing cells. Science 345(6204): 1605-1609.
- Sadasivam, S, and DeCaprio, JA (2013). The DREAM complex: master coordinator of cell cycle-dependent gene expression. Nat Rev Cancer 13(8): 585-595
doi: https://doi.org/10.1242/prelights.4637
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the developmental biology category:
Tissue mechanics and systemic signaling safeguard epithelial tissue against spindle misorientation
Ruoheng Li
Human pluripotent stem cell-derived macrophages modify development of human kidney organoids
Theodora Stougiannou
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
Also in the plant biology category:
Actin Counters Geometry to Guide Plant Cell Division
Jeny Jose
The nucleus follows an internal cellular scale during polarized root hair cell development
Jeny Jose
Conservation and divergence of regulatory architecture in nitrate-responsive plant gene circuits
Jeny Jose
preLists in the developmental biology category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
BSDB/GenSoc Spring Meeting 2024
A list of preprints highlighted at the British Society for Developmental Biology and Genetics Society joint Spring meeting 2024 at Warwick, UK.
| List by | Joyce Yu, Katherine Brown |
GfE/ DSDB meeting 2024
This preList highlights the preprints discussed at the 2024 joint German and Dutch developmental biology societies meeting that took place in March 2024 in Osnabrück, Germany.
| List by | Joyce Yu |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
2nd Conference of the Visegrád Group Society for Developmental Biology
Preprints from the 2nd Conference of the Visegrád Group Society for Developmental Biology (2-5 September, 2021, Szeged, Hungary)
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Society for Developmental Biology 79th Annual Meeting
Preprints at SDB 2020
| List by | Irepan Salvador-Martinez, Martin Estermann |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EDBC Alicante 2019
Preprints presented at the European Developmental Biology Congress (EDBC) in Alicante, October 23-26 2019.
| List by | Sergio Menchero et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |
Also in the plant biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |











 (No Ratings Yet)
(No Ratings Yet)