Collective ERK/Akt activity waves orchestrate epithelial homeostasis by driving apoptosis-induced survival
Preprint posted on 11 June 2020 https://www.biorxiv.org/content/10.1101/2020.06.11.145573v1
Article now published in Developmental Cell at http://dx.doi.org/10.1016/j.devcel.2021.05.007
Categories: cell biology
Background
The epithelium is a self-organizing tissue that coordinates cell division and cell death to maintain its barrier function (i.e. epithelial homeostasis). Cell death events continuously challenge epithelial barrier function, yet are crucial to eliminate old or critically damaged cells. Apoptotic cells can influence the fate of neighboring cells in different ways including inducing proliferation, resulting in wound-repair processes inducing further apoptosis during developmental processes requiring collective apoptosis; or triggering survival fates. These different processes imply coordination of signaling pathways that regulate proliferation, survival or apoptosis fates. However, how these signaling pathways are regulated at the single-cell level, and spatio-temporally integrated at the population level remains poorly understood.
Previous work has shown that mitogen-activated protein kinase (MAPK)/ERK-phosphoinositide-3 kinase (PI3K)/Akt signaling networks are crucial for regulation of cell fate, and play a role both at population, and single cell levels. In their work, Gagliardi et al (1) explore the roles of EGF and ERK/Akt in regulating additional single-cell fate decisions, and how the latter are integrated at the cell population level to ensure epithelial homeostasis.
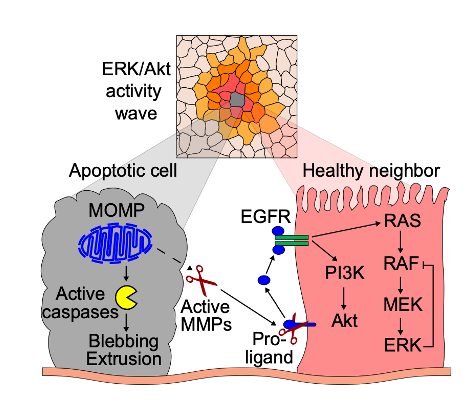
Key findings and developments
How are ERK/Akt pulses propagated from apoptotic cells to neighbouring healthy cells?
The authors generated an epithelial cell line with various reporters for Histone 2B (H2B), ERK-KTR and FoxO3a to investigate single-cell ERK/Akt activity dynamics in epithelia (the latter two biosensors report single-cell ERK and Akt activity by displaying reversible nuclear-to-cytosol translocation upon phosphorylation), and an automated image analysis pipeline to segment and track nuclei (based on H2B), and extract cytosolic to nuclear fluorescence intensities to quantify ERK/Akt activities.
They began by observing ERK/Akt pulses in starved cells, and showed that starved monolayers display spatially and temporally coordinated ERK/Akt activity pulses, resulting in collective signaling events. Such events were triggered by apoptosis resulting from the starvation-induced stress. In such case, the wave of ERK/Akt activity pulses originated from an apoptotic cell, and radiated sequentially to the subsequent layers of healthy neighbouring cells. Notably, the proportion of neighboring cells with ERK/Akt pulses sharply decreases across the layers (i.e. 90% of cells are activated in the first, 30% in the second, and 10% in the third layer).
When during the apoptosis process are ERK/Akt waves initiated?
The morphological events occurring in apoptosis include nuclear shrinkage, plasma membrane blebbing, chromatin condensation, extrusion of the apoptotic cell, nuclear fragmentation, and disaggregation into apoptotic bodies. Based on this, the authors went on to determine at what point during the sequence of apoptotic events, ERK/Akt waves were initiated. They found that the onset of ERK/Akt waves coincided with nuclear shrinkage. Moreover, they used OptoBAX, an optogenetic apoptosis actuator, to selectively induce cell death with single-cell resolution. They observed that OptoBAX-induced apoptosis triggered an ERK wave identical to the one caused by spontaneous apoptosis. To determine whether a correlation existed between ERK/Akt waves and caspase activity, they treated cells with a pan-caspase inhibitor. They found that this treatment did not prevent ERK/Akt waves neither in spontaneous events, not in OptoBAX-triggered apoptosis.
Which signaling pathways are involved in ERK/Act wave triggering?
Specific ERK or Akt inhibition resulted in abrogation of ERK or Akt, but not both, suggesting that both pathways are activated by an upstream signaling node. Based on this, the authors went on to explore upstream actuators. They inhibited EGFR catalytic activity, and showed that this completely abrogated apoptosis-triggered ERK/Akt waves, both in spontaneous events and in OptoBax-triggered apoptosis. Interestingly they showed equal effects upon antibody-based inhibition of EGFR or drug-mediated inhibition of MMP. Altogether, this suggests that ERK/Akt waves are triggered by EGFR and MMP signaling, and that they likely depend on MMP-mediated cleavage of pro-EGF ligands.
Do ERK/Akt waves regulate cell extrusion?
As a next step, the functional significance of apoptosis-triggered ERK/Akt waves was investigated. This included testing the hypothesis that ERK/Akt waves regulate the cytoskeletal process and mechanical forces participating in extrusion and/or closing of the epithelial gap. Drug-based inhibition of EGFR-dependent ERK/Akt waves, however, did not block the process of extrusion.
Do apoptosis-triggered ERK/Akt waves generate signals that regulate fate decisions?
The authors began by testing the hypothesis that ERK and Akt signaling waves provide local survival signals. For this, they generated computational approaches to analyse ERK signaling, and trained a convolutional neural network (CNN) to separate apoptotic from non-apoptotic cells based on ERK activity. First, they explored whether ERK activity dynamics differed between apoptotic cells before nuclear shrinkage, and non-apoptotic cells within the same time period. The results, based on the frequency of ERK pulses, suggest that the apoptosis/survival fates in starved monolayers depend on the ERK pulse frequency, whereby trajectories without any pulses correspond to apoptotic cells in the majority of cases, while in most trajectories with 2-5 pulses or more, cell do not undergo apoptosis. The next step was to test if different signaling dynamics in apoptotic and non-apoptotic cells specifically correlated with collective signaling events. Using computational approaches they found that collective ERK signaling events occurred mostly in non-apoptotic cells. Finally, they explored whether an ERK pulse within an apoptosis-triggered signaling wave locally promotes survival. They found that within a 4 hours time-window, secondary apoptosis is significantly less likely to occur in cells that experienced an ERK pulse induced by the primary event, suggesting that an ERK activity pulse within an apoptosis-triggered signaling wave induces survival within this time limit.
How is survival fate modulated?
Given that the results suggest that a critical pulse frequency of ERK and perhaps also of Akt are required to promote the survival fate, the authors went on to determine whether specific dynamic signaling frequencies regulate the survival fate. For this, they used two optogenetic systems to evoke synthetic signaling pulse regimes with different frequencies. One of the systems activates both ERK and Akt signaling, while the other selectively controls ERK activity only. The authors used different stimulation regimes– varying in time frequency, and evaluated apoptosis. Protection against apoptosis was observed when ERK pulses were triggered within short time regimes (3 hour intervals), but were lost within longer regimes (6 and 12 hour intervals). ERK or Akt inhibition abrogated the protection granted by optogenetic stimulation. Altogether, the results suggests that ERK signaling is sufficient to exert the pro-survival effects.
What are the physiological effects of apoptosis-induced survival?
The authors then explored whether ERK/Akt wave-mediated local survival in the vicinity of apoptotic sites contributes to endothelial homeostasis and tissue integrity in response to stress. They observed that starvation resulted in a transient peak of apoptosis that began 2-3 hours after starvation, and lasted another 2-3 until a steady-state apoptosis rate was reached. ERK/Akt activities immediately decreased with starvation and were transiently re-activated with kinetics that were slightly delayed with respect to the apoptotic rate. This suggests that the monolayer can adapt to starvation-induced stress by regulating survival to maintain homeostasis and tissue integrity.
The authors built a mathematical model consisting of two components (cells undergoing apoptosis and protection from cell death (population averaged ERK/Akt activity triggered by apoptosis) to capture the dynamic relationship between apoptosis and survival. They then explored 3 scenarios: the first scenario, considered a low rate of apoptosis and a high rate of protection (mimicking the starvation experiment) – in this case, the model agreed with the experiment, in that an episode of increased apoptosis is followed by a delayed induction of protection. In the second scenario, the rate of apoptosis was increased. The model predicted a higher protection level and apoptosis rate after the initial apoptotic event. Experimental approaches were in line with these predictions. Finally, in the third scenario, the protection rate was lowered. In this case, the apoptosis rate was higher. The experimental approaches were also in line with these predictions.
Importantly, the authors show that changes in apoptosis impact tissue integrity, as measured by holes observed in the epithelium following drug-mediated damage. These results suggest that single-cell level apoptosis-induced survival- signaling contributes to population-level tissue integrity by its ability to react to insults of different intensities, and by spatially distancing the apoptotic sites.
What I like about this preprint
I think the topic is very relevant to various fields of study within cell biology and pathology. Moreover, I found the way of approaching the questions very thorough, and the range of methods used is extraordinary – with many generated by the authors themselves. I like microscopy and image analysis, and rarely had I seen this range of techniques used in a single paper in the context of cell biology. Moreover, I enjoyed reading the work- the questions follow a very logical order, making this an enjoyable read.
References
- Gagliardi PA et al, Collective ERK/Act activity waves orchestrate epithelial homeostasis by driving apoptosis-induced survival, bioRxiv, 2020.
Posted on: 13 July 2020
doi: https://doi.org/10.1242/prelights.23041
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Fetal brain response to maternal inflammation requires microglia
Alteration of long and short-term hematopoietic stem cell ratio causes myeloid-biased hematopoiesis
Clusters of lineage-specific genes are anchored by ZNF274 in repressive perinucleolar compartments
preLists in the cell biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |











 (No Ratings Yet)
(No Ratings Yet)