Lysosomal retargeting of Myoferlin mitigates membrane stress to enable pancreatic cancer growth
Posted on: 27 January 2021
Preprint posted on 4 January 2021
Article now published in Nature Cell Biology at http://dx.doi.org/10.1038/s41556-021-00644-7
Ferlins to the rescue! Plasma membrane repair protein Myoferlin relocates to lysosomes in pancreatic cancer cells to protect lysosomes against microtears in their membrane.
Selected by Berrak UgurCategories: cancer biology, cell biology
Background:
Proper lysosomal activity is essential for regular cell function and lysosomal defects are associated with many diseases including cancer (Carmona-Gutierrez et al., 2016; Platt et al., 2012). Among cancers, pancreatic ductal adenocarcinoma (PDA) is known to rely on enhanced autophagy and lysosomal function (Perera et al., 2015; Yang et al., 2011). Although it is known that PDA cells have increased lysosomal activity, cancer specific pathways to ensure proper lysosomal function are not known.
Key Findings:
To identify lysosomal factors involved in PDA, the authors purify intact lysosomes from PDA and regular cell lines and compare the proteomic profile of these lysosomes. Through this analysis, they identify two Ferlin proteins, Myoferlin (MYOF) and Dysferlin (DysF) as the most enriched proteins in PDA lysosomes (MYOF ~30 times, DysF ~90 times enriched). The authors confirm these findings by showing that MYOF is indeed present in PDA lysosomes and bound to the lysosomal membrane. Given its known localization at the plasma membrane and its role in regulating membrane repair, the authors speculate whether Myoferlin may be targeted to lysosomes to specifically repair the lysosomal membrane (Bansal and Campbell, 2004; Bansal et al., 2003; Doherty and McNally, 2003). To test this hypothesis, they treat the lysosomes with various stressors known to impair the lysosome membrane. They observe that PDA cells last longer and retain markers of nutrient signaling in response to treatment when compared to regular pancreatic cells. The authors reason that this enhanced lysosomal protection in PDA cells may depend on MYOF, as it was highly enriched in PDA cells. To test this idea, they knock-out MYOF and show that loss of MYOF in PDA cells lead to various lysosomal defects and absence of protection against stressors. To test if MYOF-dependent lysosome protection is related to autophagy or vesicular trafficking, they knock-down several factors involved in autophagy in MYOF KO cells and observe that lysosomal defects are suppressed. Along these lines, the authors show that artificially recruiting MYOF to lysosomes in non-PDA cells protects them against stressors and that this protection is specifically regulated by the N terminal C2 domains of MYOF. Next, they confirm that MYOF expression is also increased in a mouse model of pancreatic ductal adenocarcinoma. Moreover, they report that reducing MYOF levels leads to a decrease in tumor growth in these models. Supporting these observations, they reiterate that data from The Cancer Genome Atlas show that high MYOF expression is correlated with worse survival of PDA patients. Overall, this study pinpoints MYOF as novel regulator of the lysosomal membrane and an important player in pancreatic ductal adenocarcinoma.
Take home messages:
- Myoferlin and Dysferlin are enriched on lysosomes isolated from pancreatic ductal adenocarcinoma (PDA).
- PDA lysosomes are more resistant to lysosome membrane damage
- MYOF is important for proper lysosome function in PDA cells
- Loss of MYOF leads to reduced tumor growth
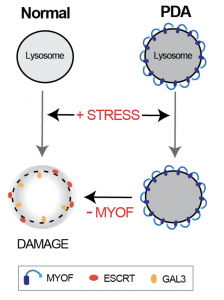
Fig.1 Model of how MYOF is regulating PDA lysosomes. MYOF is localized to lysosomes in PDA cells and regulates them agains stress. Loss of MYOF leads to membrane damage and recruitment of factors such as ESCRT and GAL3. doi: https://doi.org/10.1101/2021.01.04.425106
Why I liked this study: This preprint shows that a protein previously implicated in plasma membrane repair can localize to lysosomes to protect them against microtears in the lysosomal membrane. It is very tempting to think that MYOF has the capacity to relocate to different membranes to protect against insults. I am curious to see if MYOF or other ferlins have such a common repair role.
Questions:
- What is the partner of MYOF that recruits it to lysosomes in PDA cells? Do you speculate that it is a PDA cell specific metabolite/factor or some other commonly known factors such as Rab7? Do you think that a dominant-negative Rab7 would prevent the resilience of PDA cells against LLOMe treatment?
- MYOF overexpression is observed in different cancers in addition to pancreas adenocarcinoma (Dong et al., 2019). Do you think that your observation about MYOF’s lysosomal function in PDA is applicable to other cancer types?
- Is it possible that the suppression observed with knockdown of ATG3 or ATG7 in MYOF KO is due to relieving autophagy cargo burden? Do you think manipulating vesicular trafficking with some drugs or KD of genes known in vesicular trafficking would cause a similar suppression?
- Do you think that artificially recruiting MYOF to other organelles (such as mitochondria or peroxisomes) would prevent them against insults to their (outer) membrane?
References:
Bansal, D., and Campbell, K.P. (2004). Dysferlin and the plasma membrane repair in muscular dystrophy. Trends Cell Biol. 14, 206–213.
Bansal, D., Miyake, K., Vogel, S.S., Groh, S., Chen, C.-C., Williamson, R., McNeil, P.L., and Campbell, K.P. (2003). Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature 423, 168–172.
Carmona-Gutierrez, D., Hughes, A.L., Madeo, F., and Ruckenstuhl, C. (2016). The crucial impact of lysosomes in aging and longevity. Ageing Res. Rev. 32, 2–12.
Doherty, K.R., and McNally, E.M. (2003). Repairing the tears: dysferlin in muscle membrane repair. Trends Mol. Med. 9, 327–330.
Dong, Y., Kang, H., Liu, H., Wang, J., Guo, Q., Song, C., Sun, Y., Zhang, Y., Zhang, H., Zhang, Z., et al. (2019). Myoferlin, a Membrane Protein with Emerging Oncogenic Roles (Hindawi).
Perera, R.M., Stoykova, S., Nicolay, B.N., Ross, K.N., Fitamant, J., Boukhali, M., Lengrand, J., Deshpande, V., Selig, M.K., Ferrone, C.R., et al. (2015). Transcriptional control of the autophagy-lysosome system in pancreatic cancer. Nature 524, 361–365.
Platt, F.M., Boland, B., and van der Spoel, A.C. (2012). Lysosomal storage disorders: The cellular impact of lysosomal dysfunction. J. Cell Biol. 199, 723–734.
Yang, S., Wang, X., Contino, G., Liesa, M., Sahin, E., Ying, H., Bause, A., Li, Y., Stommel, J.M., Dell’Antonio, G., et al. (2011). Pancreatic cancers require autophagy for tumor growth. Genes Dev. 25, 717–729.
doi: https://doi.org/10.1242/prelights.27108
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cancer biology category:
Mitochondria-derived nuclear ATP surge protects against confinement-induced proliferation defects
Teodora Piskova
Spatial transcriptomics elucidates medulla niche supporting germinal center response in myasthenia gravis thymoma
Jessica Chevallier
Invasion of glioma cells through confined space requires membrane tension regulation and mechano-electrical coupling via Plexin-B2
Jade Chan
Also in the cell biology category:
Cell cycle-dependent mRNA localization in P-bodies
Mohammed JALLOH
Control of Inflammatory Response by Tissue Microenvironment
Roberto Amadio
Notch3 is a genetic modifier of NODAL signalling for patterning asymmetry during mouse heart looping
Bhaval Parmar
preLists in the cancer biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Anticancer agents: Discovery and clinical use
Preprints that describe the discovery of anticancer agents and their clinical use. Includes both small molecules and macromolecules like biologics.
| List by | Zhang-He Goh |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
Also in the cell biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |











 (No Ratings Yet)
(No Ratings Yet)