Repairing neural damage in a C. elegans chemosensory circuit using genetically engineered synapses
Posted on: 17 May 2020
Preprint posted on 17 April 2020
Article now published in Cell Systems at http://dx.doi.org/10.1016/j.cels.2020.12.003
Unexpected bonds, help to repair and respond: Artificially expressed electrical synapses in C. elegans neurons strengthen weak signals and restore circuit function and behaviour.
Selected by Sahana SitaramanCategories: bioengineering, developmental biology, neuroscience
Background:
Behind every animal behaviour lies the metaphorical guiding hand of a neural circuit. For many decades, neuroscientists have worked towards understanding how neurons wire together to form functional circuits and how these circuits help execute and modulate behaviours. A widely used approach to studying circuit function involves tinkering with the component neurons and their activity, using techniques such as cell ablation[1] and optogenetics[2]. But recent advances in synthetic neurobiology have allowed researchers to alter circuit wiring by introducing specific synaptic connections and thereby reprogram the associated behavioural output[3].
Remodelling circuit architecture by introducing artificial links can not only help tease apart the roles of different circuit modules, but also repair them in case of synaptic loss or neuronal degeneration. Due to redundancy present in circuit configurations, rerouting information flow by introducing engineered synapses could help restore signal transmission and normal behaviour. This study uses the well described Caenorhabditis elegans (roundworm) nervous system[4], particularly the chemosensory circuit, to test this possibility. The authors use simple experiments to check if expressing engineered electrical synapses in a damaged circuit helps restore information flow and rescue chemotaxis behaviour.
Key Findings:
In 1986, John Graham White described the entire structure of C. elegans nervous system using electron microscopic reconstruction[4]. The high-resolution images allowed White and his colleagues to identify and map out the complete set of 302 neurons, their connections and all the neuronal circuits. Due to the immense level of detail available for the C. elegans nervous system and its amenability to genetic manipulations, it serves as the perfect model system to observe circuit repair by synthetic synapses.
The authors chose the well characterised chemosensory circuit for their study, which comprises of a pair of AWC odor sensory neurons, sending parallel projections to multiple interneuron classes like AIA and AIB, which are also present in pairs (Figure 1). While structurally similar, the left and right AWC are functionally distinct, sensing different odors. But both sense isoamyl alcohol, the chemical used in the experimental assays by the authors.
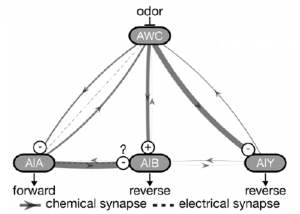
To determine how altering this circuit would affect its functioning, the researchers removed the AIA neurons and observed the activity AIB neurons. Absence of AIA strongly diminished AIB response, to both isoamyl alcohol presentation and removal. This change also had downstream effects, causing a reduction in chemotaxis of the worms towards the odor source. What was interesting was a further decrease in chemotaxis on removal of both AIA and AIB, suggesting that AIB cells still maintained some level of functioning, possibly due to inputs coming from AWC.
Having established a system where removal of a neuron type affected the proper operation of the circuit, the authors probed the effects of inserting genetically engineered electrical synapses into the system. This was achieved by expressing the mammalian Connexin36 (Cx36) electrical synapse protein in AWC and AIB neurons, with the expectation that the signal transmission between them would be strengthened, compensating for the lack of AIA to AIB connection. As expected, expressing Cx36 in both neuron types led to a rescue in chemotaxis, even exceeding wildtype levels. But surprisingly, expressing Cx36 in either neuron type also rescued the behaviour. This could be explained by lateral electrical coupling between left and right AWC or AIB neuron types, leading to an enhancement of weak signals.
To test this hypothesis, the authors tested the response of AWC neurons to low concentration of isoamyl alcohol, with and without Cx36 expression. Cx36 mediated coupling between AWC neurons caused an upward shift in their response to the same concentration of the odor, essentially strengthening a weak stimulus. This was corroborated by observing the activity of AIB neurons with ectopic Cx36, in the absence of AIA cells. Coupled AIB neurons showed an enhanced response to odor, despite the removal of AIA.
Lastly, to weigh the effects of AWC to AIB coupling versus lateral coupling, the team expressed Cx36 only in one of the AWC neurons along with AIB neurons. They observed
that the recovery in chemotaxis for the above experiment was significantly more than when Cx36 was expressed only in AIB neurons. This suggests a critical role for AWC-AIB coupling in behavioural recovery of the worm.
Using uncomplicated experiments, the authors have been successful in demonstrating how engineered synapses can be used to restore the information transfer within a damaged neuronal circuit. They have also been triumphant in showing the functional recovery of the circuit as well as the behaviour it controls.
What I liked about the preprint:
I found this study to be extremely innovative. While there are many approaches to circuit repair, like brain grafts, stem cells[5], and magnetic stimulation[6], this is one of the few studies targeting the problem at a synaptic level. Their use of electrical coupling to overcome the absence of chemical synapses is clever and easy to translate to other systems.
Electrical synapses are generally overlooked in our pursuit to understand the brain. So, the focus on exploring their benefits and contributions to circuit function was another reason I enjoyed this preprint.
Questions for the authors:
- How do you propose to repair the chemosensory circuit to respond to odors like butanone and 2,3-pentanedione which activate either AWC-ON or AWC-OFF neuron?
- How translatable is this method of circuit recovery towards therapeutic advances for neurodegenerative diseases like Alzheimer’s disease?
- Keeping in mind the massive role glia play in synapse formation and pruning, have you considered coupling glial cells to reprogram circuits?
References:
1. Marquart GD, Tabor KM, Bergeron SA, Briggman KL, Burgess HA (2019) Prepontine non giant neurons drive flexible escape behavior in zebrafish. PLoS Biol
2. Zhu P, Narita Y, Bundschuh S, Fajardo O, Schärer Y-PZ, Chattopadhyaya B, Bouldoires EA, Stepien AE, Deisseroth K, Arber S, Sprengel R, Rijli FM and Friedrich RW (2009). Optogenetic dissection of neuronal circuits in zebrafish using viral gene transfer and the Tet system
3. Rabinowitch, I. et al (2014) Rewiring neural circuits by the insertion of ectopic electrical synapses in transgenic C. elegans. Nat. Commun
4. White, J., Southgate, E., Thomson, J. & Brenner, S (1986) The structure of the nervous system of the nematode Caenorhabditis elegans. Phil. Trans. R. Soc. Lond. (Biol.)
5. Jessberger, Sebastian (2016) “Neural repair in the adult brain.” F1000Research
6. T. Dufor, S. Grehl, A. D. Tang, M. Doulazmi, M. Traoré, N. Debray, C. Dubacq, Z.-D. Deng, J. Mariani, A. M. Lohof, R. M. Sherrard (2019) Neural circuit repair by low-intensity magnetic stimulation requires cellular magnetoreceptors and specific stimulation patterns. Sci. Adv.
doi: https://doi.org/10.1242/prelights.20727
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the bioengineering category:
Scalable and efficient generation of mouse primordial germ cell-like cells
Carly Guiltinan
Generalized Biomolecular Modeling and Design with RoseTTAFold All-Atom
Saanjbati Adhikari
Multi-pass, single-molecule nanopore reading of long protein strands with single-amino acid sensitivity
Benjamin Dominik Maier, Samantha Seah
Also in the developmental biology category:
Gestational exposure to high heat-humidity conditions impairs mouse embryonic development
Girish Kale, preLights peer support
Modular control of time and space during vertebrate axis segmentation
AND
Natural genetic variation quantitatively regulates heart rate and dimension
Girish Kale, Jennifer Ann Black
Notch3 is a genetic modifier of NODAL signalling for patterning asymmetry during mouse heart looping
Bhaval Parmar
Also in the neuroscience category:
Sexually dimorphic role of diet and stress on behavior, energy metabolism, and the ventromedial hypothalamus
Jimeng Li
Enhancer-driven cell type comparison reveals similarities between the mammalian and bird pallium
Rodrigo Senovilla-Ganzo
Autism gene variants disrupt enteric neuron migration and cause gastrointestinal dysmotility
Rachel Mckeown
preLists in the bioengineering category:
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Advances in microscopy
This preList highlights exciting unpublished preprint articles describing advances in microscopy with a focus on light-sheet microscopy.
| List by | Stephan Daetwyler |
Also in the developmental biology category:
BSDB/GenSoc Spring Meeting 2024
A list of preprints highlighted at the British Society for Developmental Biology and Genetics Society joint Spring meeting 2024 at Warwick, UK.
| List by | Joyce Yu, Katherine Brown |
GfE/ DSDB meeting 2024
This preList highlights the preprints discussed at the 2024 joint German and Dutch developmental biology societies meeting that took place in March 2024 in Osnabrück, Germany.
| List by | Joyce Yu |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
2nd Conference of the Visegrád Group Society for Developmental Biology
Preprints from the 2nd Conference of the Visegrád Group Society for Developmental Biology (2-5 September, 2021, Szeged, Hungary)
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Society for Developmental Biology 79th Annual Meeting
Preprints at SDB 2020
| List by | Irepan Salvador-Martinez, Martin Estermann |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EDBC Alicante 2019
Preprints presented at the European Developmental Biology Congress (EDBC) in Alicante, October 23-26 2019.
| List by | Sergio Menchero et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |
Also in the neuroscience category:
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |











 (No Ratings Yet)
(No Ratings Yet)