Thyroid hormone regulates distinct paths to maturation in pigment cell lineages
Posted on: 5 March 2019
Preprint posted on 22 January 2019
Article now published in eLife at http://dx.doi.org/10.7554/eLife.45181
Thyroid hormone regulation of zebrafish pigment cells – not a black and white (or yellow) issue
Selected by Hannah BrunsdonCategories: cell biology
Background
How zebrafish pigment cells develop, differentiate and organise themselves into their famous stripe pattern has interested researchers from a developmental and evolutionary perspective for decades. There are three pigment cell types in zebrafish: black melanophores (analogous to mammalian melanocytes) yellow xanthophores, and silvery iridophores. All three lineages are derived from a common neural crest (NC) progenitor, and during development they proliferate and differentiate to pattern the embryo. In post-embryonic stages, embryonic xanthophores proliferate and enter a ‘cryptic phase’ where they lose their visible pigmentation. Later, during stripe formation, they re-differentiate and regain their pigmentation. Adult melanophores and iridophores however, are provided from a post-embryonic stem cell pool[1].
Previously, the Parichy lab showed how alterations in thyroid hormone (TH) levels disrupted post-embryonic pigment cell development and patterning. Fish lacking TH because of transgenic thyroid ablation or genetic mutation lacked visible xanthophores but had supernumerary melanophores[2]. In this preprint, Saunders et al explore two hypotheses that might explain the mechanism of this opposing action of TH on melanophores and xanthophores – 1) that TH influences cell specification and directs multipotent cells from one fate towards the other, or that 2) TH has a discordant effect, selectively amplifying one lineage whilst repressing the other.
Key findings
First, the authors performed scRNA-seq on isolated wild type (euthyroid) and thyroid ablated hypothyroid zebrafish skin cells labelled by the sox10:Cre, which marks NC derivatives. Samples were collected from multiple stages of larval to adult development to capture the entire formation of adult patterning. Clustering analyses identified a variety of NC-derived cell types, including melanophores, xanthophores and iridophores as well as multipotent pigment progenitor cells shown by the UMAP plot below:
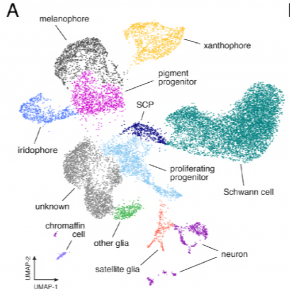
From preprint Figure 2: Single cell transcriptomic identification of post-embryonic NC-derived cell types from euthyroid and hypothyroid fish.
These analyses also enabled the identification of novel iridophore and xanthophore markers and gave some surprising insights into the distribution of well-known lineage markers – for example, the melanophore master regulator transcription factor mitfa is also expressed appreciably in xanthophores.
Next, cells were ordered pseudotemporally to investigate how transcriptional dynamics change during pigment cell lineage maturation from a common progenitor. Branched expression analysis modelling (BEAM) analysis confirmed that genes involved in lineage specification such as mitfa were expressed early in pseudotime, whereas those like dct involved in differentiation were expressed later. Interestingly, this analysis also revealed the differential expression of many novel genes at different points in pseudotime, suggesting that they may be important for discrete steps in lineage-specific maturation.
So, how are these processes influenced by thyroid hormone? The authors’ first hypothesis that TH directs cells away from one fate and towards the other was rejected, because the clusters and trajectory topologies of euthyroid and hypothyroid cells were equivalent, and there was no evidence of either lineage being depleted. The second hypothesis of ‘lineage discordance’ was also disproved, as the distribution of both melanophores and xanthophores were biased towards early pseudotime in hypothyroid fish, arguing against a model where TH promotes xanthophore differentiation while blocking melanophore differentiation.
These findings led the authors to consider a third hypothesis, that TH might promote the maturation of both lineages, but in different ways to produce the observed differences in cell numbers in hypothyroid fish. Indeed, melanophores in euthyroid fish were more likely to be binucleate and have increased lysosomal staining, both of which are characteristic of terminally differentiated melanophores. This experimental evidence, supported by increases in the expression of melanocyte maturation signatures over pseudotime in the scRNA-seq data, suggests that TH promotes melanophore maturation.
TH was also found to promote xanthophore maturation. The yellow pigment in xanthophores is made from pteridine- and carotenoid-based pigment molecules. Interestingly, the authors showed through HPLC analysis that carotenoids were present in euthyroid, but not hypothyroid fish skin. This is reminiscent of the zebrafish scarb1 mutant phenotype, as fish without scarb1 – essential for cellular uptake of carotenoids – lack mature yellow interstripe xanthophores, even though these cells have wild-type levels of pteridines during embryonic development. Importantly, the authors showed that this scarb1 phenotype was rescued after treatment with exogenous TH, as shown below:
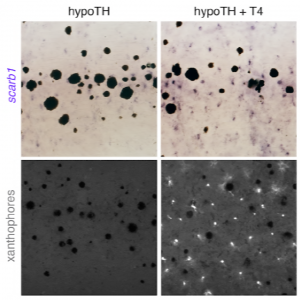
From preprint Figure 6, supplement 3. Scarb1 mutants lack mature xanthophores as shown by a lack of carotenoid autofluorescence. However, this is rescued within two days after the addition of exogenous TH (T4).
Therefore, the authors concluded that during xanthophore maturation, TH regulates a switch to primarily carotenoid-based colouration and is therefore required for xanthophore re-pigmentation during adult development.
Taken together, this confirms the authors’ third hypothesis, that TH promotes the maturation of both xanthophore and melanophore lineages. Without TH, melanophores derived from the post-embryonic stem cell niche are stuck in a transit-amplifying phase, and cryptic xanthophores poised to re-differentiate cannot activate carotenoid pigmentation.
Why I chose this preprint
Recent single cell RNA-seq based papers in the zebrafish have been really useful in exploring the intricacies of gene expression dynamics as cells commit to specific lineages and differentiate during early zebrafish embryonic development. However, not as much is known about post-embryonic zebrafish development, including how pigment cell lineages continue to be regulated at the transcriptional level. Therefore, I was drawn to this preprint even without the hypothyroid aspect of the work. I also liked how their RNA-seq analyses uncovered a new “pteridine-to-carotenoid switch” in maturing xanthophores which was backed up really nicely with experimental work.
Questions for the authors
- This is probably well outside the scope of the paper, but I find it really interesting that xanthophores utilise two different pigment bases to make yellow pigment at different stages of development. Are these two yellow colours different shades, and what might be the evolutionary advantage of having two ways of pigmenting xanthophores? It is surprising to me that pteridine production does not noticeably compensate for a lack of carotenoids.
- Figure 3 supplement 1 shows there are distinct subpopulations of xanthophores. Do these populations cluster on whether TH is present, or if carotenoid or pteridine-associated genes predominate, or if they are pigmented or cryptic? Connected to this, how does one characterise or visualise a cryptic xanthophore, assuming they do not autofluoresce – do they have a distinct transcriptional signature to pigmented xanthophores?
- In the hypothyroid phenotype, it looks like melanophores encroach into the xanthophore interstripe domain instead of proliferating within their normal stripe region – do you see TH affecting genes involved in pigment cell migration/patterning or lateral inhibition?
References
- Budi EH, Patterson LB, Parichy DM. 2011. Post-embryonic nerve-associated precursors to adult pigment cells: genetic requirements and dynamics of morphogenesis and differentiation. PLoS Genet 7:e1002044.
- McMenamin SK, et al. 2014. Thyroid hormone-dependent adult pigment cell lineage and pattern in zebrafish. Science 345:1358–1361.
doi: https://doi.org/10.1242/prelights.9177
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Resilience to cardiac aging in Greenland shark Somniosus microcephalus
Theodora Stougiannou
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
preLists in the cell biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
December in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cell cycle and division 2) cell migration and cytoskeleton 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Matthew Davies et al. |
November in preprints – the CellBio edition
This is the first community-driven preList! A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. Categories include: 1) cancer cell biology 2) cell cycle and division 3) cell migration and cytoskeleton 4) cell organelles and organisation 5) cell signalling and mechanosensing 6) genetics/gene expression
| List by | Felipe Del Valle Batalla et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |











 (No Ratings Yet)
(No Ratings Yet)