Nuclear stiffness decreases with disruption of the extracellular matrix in living tissues
Posted on: 2 December 2020
Preprint posted on 31 August 2020
Article now published in Small at http://dx.doi.org/10.1002/smll.202006699
Categories: biophysics, cell biology
Background
Reciprocal interactions between the cell nucleus and the extracellular matrix lead to macroscale tissue phenotype changes. Changes in cell gene expression lead to alterations in signalling that regulate cell communication, timing of cellular activities and tissue structure. The extracellular matrix dictates another layer of cellular regulation, as biochemical cues, physical forces, and changes in stiffness of the extracellular matrix microenvironment guide cell migration, proliferation, differentiation, and changes in gene expression. The extracellular environment is physically linked to the nuclear envelope and provides cues to maintain nuclear structure and cellular homeostasis regulated, partly, by mechano-transduction mechanisms. Cells are physically linked to their local matrix via focal adhesions, and this allows cells to respond to their physical environment. Within the cell, the cytoskeleton is connected to the nucleus through the LINC (linker of nucleoskeleton and cytoskeleton) complex. This, variations in extracellular matrix mechanics are propagated to the cell nucleus affecting cellular processes such as protein conformation, localization of transcription factors and chromosome organization. The nucleus regulates homeostasis and cell phenotype partly through mechano-transduction mechanisms. The nuclear envelope acts as a shock absorber to maintain nuclear architecture when the cell environment changes. Altogether, studying the mechanical properties of the nucleus can ultimately provide insight into changes in gene regulation responsible for changes in cell phenotype and cell pathology. Although in vitro assays exist to study nuclear mechanics, these do not allow studying the complex interplay between extracellular matrix, cell and nuclear stiffness in the context of a tissue. To address this gap of knowledge, in their work McCreery et al (1) present a method that combines atomic force (AFM) and fluorescence confocal microscopy, and enables investigating the impact of extracellular matrix degrading enzymes on nuclear mechanics while cells are embedded in their native tissue environment.
Key findings and developments
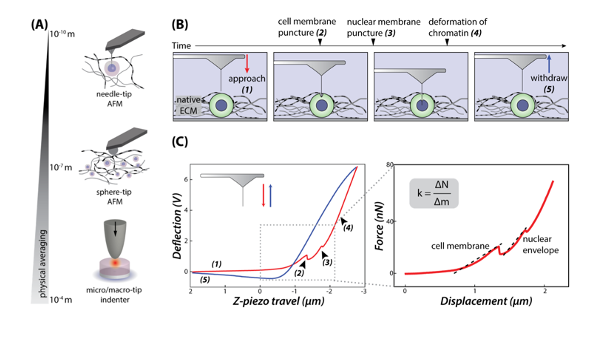
The authors begin by reporting a method to directly measure nuclear membrane stiffness while maintaining cell-matrix interactions. They go on to investigate whether biomechanical disruption of tissues is transmitted to the cell and nucleus, and how the responses of the nucleus to the ECM can be measured by AFM. The experiments showed that enzymatic treatment of cartilage tissue explants causes softening of nuclei within embedded cells, and that the AFM needle-tip technique allows distinguishing local extracellular matrix, cell membrane, and nuclear membrane stiffness. Fluorescence microscopy is used to visualize cell and nuclear structures and to align the needle tip over the desired structures. The authors were able to report stiffness values of the cell membrane and nuclear envelope by fitting the force-displacement data before relaxation of a corresponding needle puncture to a linear model. To validate needle penetration into the cell and nuclear structures, the authors mounted the AFM system onto an inverted laser scanning confocal microscope to observe the force spectroscopy curve, and to image fluorescence of isolated cells. In order to distinguish membrane deformation and membrane puncture, the authors used reporter HeLa cells stably expressing a charged multivesicular body protein 4B (CHMP4B-GFP). They then report differences between HeLa cell and chondrocyte puncture in terms of membrane stiffness, and attribute this to the small distance between the cell and nuclear membranes in HeLa cells causing the membrane to deform and evenly contact the nuclear envelope before puncturing both. They suggest this finding shows a limitation of the AFM needle-tip technique because the nuclear membrane stiffness may not be resolved in cells that spread on culture plates or have plasma membranes that are easily deformable. The authors then used a sphere-tip probe to indent cell membrane structures, and showed little change of fluorescence intensity at the site of measurements, confirming that membrane integrity is not disrupted by a rounded tip. Altogether, the results show that cell functionality and viability are preserved during and immediately after needle penetration, indicating that the AFM needle-tip technique is compatible with live cells and useful for nuclear probing.
The authors then investigated the impact of biochemical degradation of bovine cartilage tissue on ECM, cell, and nuclear stiffness when treated over time with cartilage degrading enzymes (ADAMTS4-responsible for the pathological cleavage of aggrecan, and MMP13-which cleaves a range of type II collagen peptides). They measured these effects using the AFM needle tip technique combined with fluorescence. They used tissue sections parallel to the cartilage surface to expose middle zone chondrocytes. They then probed the cell structures with AFM, measuring extracellular matrix stiffness outside of the chondron (i.e. the local extracellular matrix to the cell). Since chondrocytes regulate extracellular enzymes, and enzyme activity occurs in the extracellular space, the authors suggest that cell and nuclear stiffness values obtained are probably reflective of extracellular matrix mechanics and not a direct result of cellular exposure to MMP13 and ADAMTS4. Disruption of the cartilage extracellular matrix by ADAMTS4 results in decreased stiffness of cartilage, cell membrane and nuclear envelope of embedded chondrocytes. Equally, biochemical disruption of the matrix by MMP13 reduces the stiffness of the cartilage tissue, local matrix, cell membrane and nuclear envelope. For both enzymes, as they disrupt extracellular matrix molecules and reduce its foundational structure, a cellular response is reduction of nuclear stiffness. An important difference between both enzymes is the timing and extent by which nuclear stiffness variation is observed. The authors suggest that these discrepancies provide insight into a) the propagation of matrix signals to the cell nucleus and b) the impact of disrupting different functional components of cartilage. Altogether, outcomes with both enzymes demonstrate that disruption of the cartilage matrix destabilizes cartilage structure and the transfer of intrinsic forces within the tissue, which may be transmitted to the cell surface. Moreover, disruption of links between the extracellular matrix to the cell nucleus contributes to pathology by triggering chromatin rearrangement and having an effect on gene expression. The authors finalize by suggesting that studied on nuclear stiffness should not be limited to quantification of nuclear mechanics alone or nuclear mechanics in living cells, but should include interactions of the nuclear envelope with the extracellular prestress among tissue types. Moreover they propose the method hereby presented as the basis for future studies of nuclear elastography of cells within living tissues, and indicate that specific challenges will arise and should be addressed, depending on the tissue type studied.
What I like about this preprint
I particularly like studies that explore gaps of knowledge, and generate methods that make it possible for the scientific community to study biological phenomena in a context as close to the true environment as possible. I also like that the authors address a topic in an interdisciplinary manner, which I think also has led to important discoveries and methods widely used by the scientific community.
Open questions
- You mention that several challenges remain, specific to certain tissue types, and briefly mention a limitation of your method under specific conditions. Can you expand more on what other potential challenges different tissues might pose, and how/if your method can circumvent those challenges?
- Specific to cells, you discussed the differences you observed between HeLa cells and chondrocytes. For experimental setups, have you found whether primary cells are very different from cell lines? Are you considering generating a “map” of multiple cells representative of various possible tissues?
- Did you investigate the changes in gene expression of the various cells after being submitted to changes in the extracellular matrix caused by the degrading enzymes you used in your study? And further, how and if those gene expression changes impact cell morphology or pathology?
- Following from the question above, is there a threshold stress level in the nuclear envelope whereby no changes to chromatin organization occur in the nucleus?
- As future directions, do you envisage the AFM needle tip technique to be used in the context of, for instance, cancer and infection biology?
- How compatible is the method with multiple other imaging setups- that is, how many hybrid measurements (considering multiple scales) could be done in an integrated setup?
References
- McCreery et al , Nuclear stiffness decreases with disruption of the extracellular matrix in living tissues, bioRxiv, 2020.
doi: https://doi.org/10.1242/prelights.26107
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the biophysics category:
Active flows drive clustering and sorting of membrane components with differential affinity to dynamic actin cytoskeleton
Teodora Piskova
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
Mitochondrial Fatty Acid Oxidation is Stimulated by Red Light Irradiation
Rickson Ribeiro, Marcus Oliveira
Also in the cell biology category:
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
Kosmos: An AI Scientist for Autonomous Discovery
Roberto Amadio et al.
preLists in the biophysics category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
66th Biophysical Society Annual Meeting, 2022
Preprints presented at the 66th BPS Annual Meeting, Feb 19 - 23, 2022 (The below list is not exhaustive and the preprints are listed in no particular order.)
| List by | Soni Mohapatra |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
Biophysical Society Meeting 2020
Some preprints presented at the Biophysical Society Meeting 2020 in San Diego, USA.
| List by | Tessa Sinnige |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Biomolecular NMR
Preprints related to the application and development of biomolecular NMR spectroscopy
| List by | Reid Alderson |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
Also in the cell biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
December in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cell cycle and division 2) cell migration and cytoskeleton 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Matthew Davies et al. |
November in preprints – the CellBio edition
This is the first community-driven preList! A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. Categories include: 1) cancer cell biology 2) cell cycle and division 3) cell migration and cytoskeleton 4) cell organelles and organisation 5) cell signalling and mechanosensing 6) genetics/gene expression
| List by | Felipe Del Valle Batalla et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |











 (No Ratings Yet)
(No Ratings Yet)