A Method to sort heterogenous cell populations based on migration in 2D and 3D environments
Preprint posted on 7 May 2020 https://www.biorxiv.org/content/10.1101/2020.05.06.080234v1
Categories: biophysics, cell biology
Background
Cell migration plays a pivotal role in all stages of the life of a multicellular organism. Important homeostatic processes requiring cell migration include tissue morphogenesis, wound healing and immune responses. Conversely, aberrant migration of diseased cells, such as that displayed by cancer cells, can result in metastasis. Phenotypic assays, including Boyden chambers and wound healing assays, have been developed to study the migratory potential of cells at the population level. Equally, quantitative high-resolution imaging has allowed the study of the molecular basis of collective cell migration. Approaches to study cell migration in 3D are less popular due to the technical challenges of imaging. Despite their utility, although various of these 2D and 3D assays reveal heterogeneity of migratory behavior within a cell population, the quantitative analysis usually provides only a migration index averaged over the entire cell population, and many of them do not provide easy means to sort and retrieve cells from within the population. Altogether, very few methods exist that can sort subpopulations of cells based on their migratory behaviour from an initial heterogeneous pool. In their work, Arora et al present a new approach to sort migratory cancer and immune cells, based on their spontaneous migration in 2D and 3D microenvironments (1).
Key findings and developments
In their work, Arora et al propose readily implementable methods to separate a faster migrating sub-population of cells from a heterogeneous population based on migration in 2D or 3D environments. Initially unsorted groups of cells are locally confined in a series of scattered predefined regions. They are then left to migrate spontaneously away from the original confinement zone to another substrate (for 2D or 3D as described below).
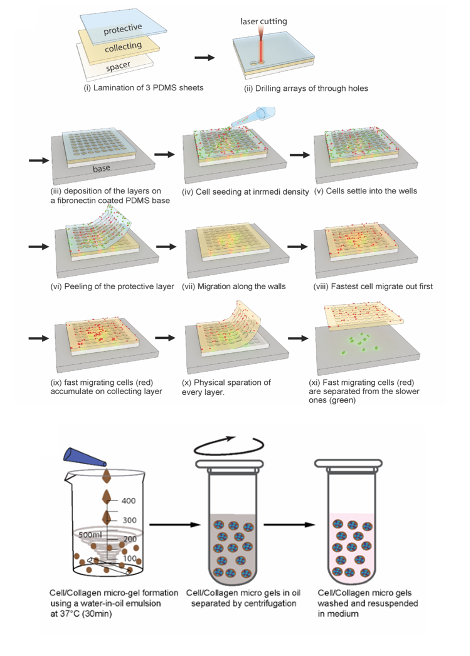
2D sorting device and proof of concept
The 2D migration sorting assay (2D-MSA) relies on a multi-layered PDMS micro-well device, in which arrays of holes are perforated, using a layer cutter. Cells are seeded at 70-80% confluency, and some of these cells will fall into the perforated cavities, and will adhere within a short time. Cells are then allowed to migrate up the cavity walls, to reach the top collection layer initially devoid of any cells. The authors optimized the diameter and height of the cavities, as well as the spacing between cavities, to accurately study cell migration. Ultimately, the collection layer will be enriched with fast migrating cells, while the base layer will be enriched in slow migrating cells. The authors validated the method using a 1:1 mixture of cell lines MCF7 and MD MB 231. Both are breast cancer derived cell lines, however, they have different motility characteristics: while MCF7 maintains an epithelial state and lacks the ability to metastasize, the latter is mesenchymal, with extensive migratory capacity. Both were differentially labeled for recognition. Quantification of both cell lines using image analysis revealed a significant enrichment of MDA MB 231 cells on the top layer of the device, as expected. This demonstrated the 2D-MSA to be useful for separating a heterogeneous cancer cell population into a more homogeneous population based on migration. The authors went on to further test the method with patient samples, and performed downstream analyses on the different sorted cell populations.
3D sorting device and proof of concept
The 3D sorting uses hierarchical hydrogel systems consisting of collagen micro-gels suspended in degradable bulk hydrogel. The surrounding matrix is rapidly cross-linked while mixing to minimize the sedimentation of the collagen beads. The way this method can be used to separate cancer cells in 3D, is that cells are left to migrate for several days from the collagen microbeads into the cleavable matrix. The cleavable matrix can then be selectively digested to release all the migrated cells, and a strainer can be used to separate the collagen beads from the less mobile cells. Each fraction can then be cultured separately. As for the 2D device testing, the authors used a 1:1 mixture of cell lines MCF7 and MD MB 231 as proof of concept, and again demonstrated that this method allows separation of both populations. They went on to test their method on cell types for which 2D migration assays are challenging, such as primary leukocytes, in this case, T cells. Using 3D sorting, they achieved a substantial enrichment of fast migrating cytotoxic T lymphocytes. Downstream analyses showed that these faster migrating T cells had significantly higher killing efficiency as compared to slow migrating, and unsorted cells. This further supports the potential of the method, for functional sorting of CTLs in the context of immunotherapeutic applications.
The authors further emphasize the easy implementation of this method, its high throughput, and the fact that it allows downstream genomic, molecular, phenotypic and functional tests.
What I like about this preprint
I like the out-of-the-box approach for solving a methodological problem that has existed for a long time. It’s ingenious, uses the cell’s own biophysical properties, and allows downstream analyses in the sorted cells. It overcomes many of the limitations of other existing assays, and is in many cases complementary. I like the proofs of concept, and the fact that the authors included a very detailed materials and methods section which allows reproducibility of the devices.
References
- Arora A., et al A method to sort heterogeneous cells populations based on migration in 2D and 3D environments, bioRxiv, 2020.
Posted on: 2 July 2020
doi: https://doi.org/10.1242/prelights.22587
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the biophysics category:
Structural basis of respiratory complexes adaptation to cold temperatures
Actin polymerization drives lumen formation in a human epiblast model
Learning a conserved mechanism for early neuroectoderm morphogenesis
Also in the cell biology category:
Fetal brain response to maternal inflammation requires microglia
Alteration of long and short-term hematopoietic stem cell ratio causes myeloid-biased hematopoiesis
Clusters of lineage-specific genes are anchored by ZNF274 in repressive perinucleolar compartments
preLists in the biophysics category:
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
66th Biophysical Society Annual Meeting, 2022
Preprints presented at the 66th BPS Annual Meeting, Feb 19 - 23, 2022 (The below list is not exhaustive and the preprints are listed in no particular order.)
| List by | Soni Mohapatra |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
Biophysical Society Meeting 2020
Some preprints presented at the Biophysical Society Meeting 2020 in San Diego, USA.
| List by | Tessa Sinnige |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Biomolecular NMR
Preprints related to the application and development of biomolecular NMR spectroscopy
| List by | Reid Alderson |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
Also in the cell biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |











 (No Ratings Yet)
(No Ratings Yet)