Hyaluronic Acid and Emergent Tissue Mechanics Orchestrate Digit Tip Regeneration
Posted on: 20 March 2025
Preprint posted on 8 December 2024
Mui and team show hyaluronic acid deposition and a soft ECM promote digit regeneration in mice.
Selected by Jonathan TownsonCategories: biophysics, developmental biology, physiology
Background
If we suffer the loss of the tip of our finger, it will grow back. However, we lack the ability to regenerate any more than this, for example, if we lose an arm then it doesn’t come back. As well as being an interesting developmental biology problem, a better understanding of the mechanisms controlling regeneration could also enable better therapeutic treatment of wounds (Simkin et al., 2015; Storer & Miller, 2021).
Humans are not completely lacking in regenerative ability, for example foetal wounds heal/regenerate without scarring (Moore et al., 2018). Unlike foetal wounds, adult injuries heal through deposition of extracellular matrix (ECM) components in a process called fibrosis, eventually leading to scar formation (Wynn & Ramalingam, 2012). In these examples, hyaluronic acid (HA) is key in the foetal wound repair, whilst fibrotic wounds typically get much stiffer (Mast et al., 1992; Ziol et al., 2005). The ECM substrate can therefore play a vital role in the regenerative capability of a wound, and changes in the mechanical environment affect tissue repair and regeneration (Kuehlmann et al., 2020; Rosińczuk et al., 2016). Nevertheless, by comparison to molecular and cellular signals, mechanical cues from the ECM are an understudied area which has been of increasing interest.
In this first preprint from the Storer lab, Mui and colleagues delve into the importance of the ECM in wound regeneration in mice, revealing that soft substrates and HA deposition promote regeneration.
Key findings
Summary
The team used a mouse model of regeneration where digits were amputated to generate regenerative (blastema) or non-regenerative wounds. They first compared the two wound types and found blastemas to be softer and have higher levels of HA deposition compared to non-regenerative wounds which were stiffer, fibrotic and had more collagen. They then experimentally challenged the system by preventing HA synthesis and showed HA levels can determine wound stiffness and regenerative capability, and that HA levels and substrate stiffness also affect BMP signalling. Finally, they showed HAPLN1 is mechanosensitive and can stabilise HA, with overexpression restoring regenerative capacity.
Blastemas have a softer ECM environment compared to non-regenerative wounds
First, the team applied second harmonic generation microscopy (SHGM) and atomic force microscopy (AFM) to image Blastema and non-regenerative wounds (Fig. 1). SHGM is label free non-invasive and especially good for labelling collagen and muscle structures (Aghigh et al., 2023; Chen et al., 2012). Whilst AFM uses a cantilever to scan over the tissue at a set pressure and measure the substrate stiffness.
Using these novel techniques on blastemas and non-regenerative wounds, the authors demonstrated that the blastema has less collagen deposition and is softer than the non-regenerative wounds.
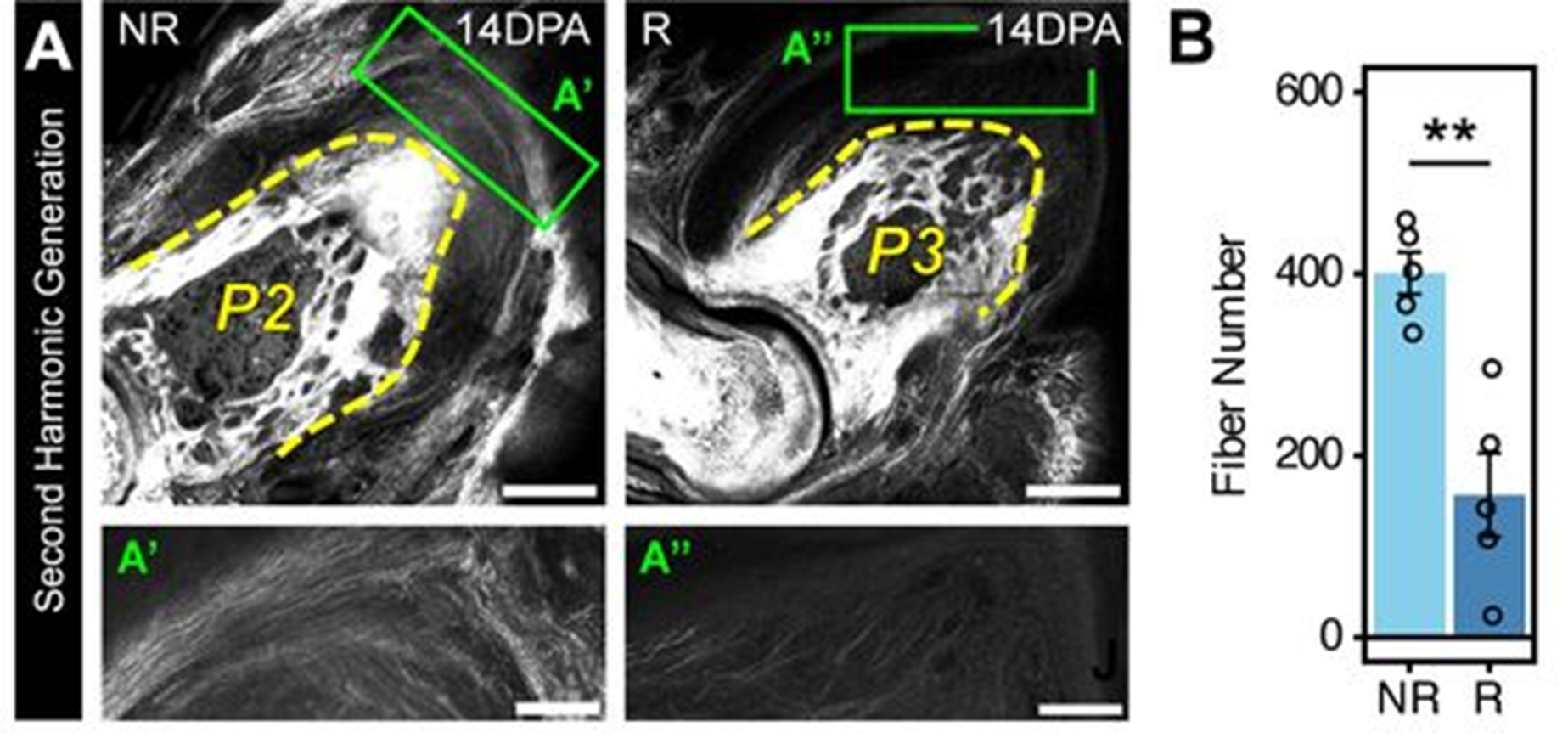
Figure 1: Second harmonic generation microscopy from figure 1 of Mui et al (2024) showing greater collagen deposition in non-regenerative (NR) than regenerative (R) wounds. Image made available under a CC-BY-NC-ND 4.0 International license.
The composition of fibroblast populations differs between Blastema and non-regenerative wounds
Fibroblasts are the principal cell type in connective tissue and have functional heterogeneity within their population (Ganier et al., 2022; Lendahl et al., 2022). Here, Mui and colleagues performed single cell sequencing and identified fibroblasts based on expression of PDGFRA (Fig. 2). They found that two populations (fibroblast 1 and 2) deposit collagen and contribute to a stiff environment. However, in the blastema, an osteo-lineage (OL) fibroblast population synthesises hyaluronic acid (HA), Hyaluronan and Proteglycan Link Protein 1 (HAPLN1), and aggrecan (ACAN). This OL population is three times more prevalent in Blastema than non-regenerative wounds and the synthesis of HA, HAPLN1 and ACAN form a distinct ECM niche.
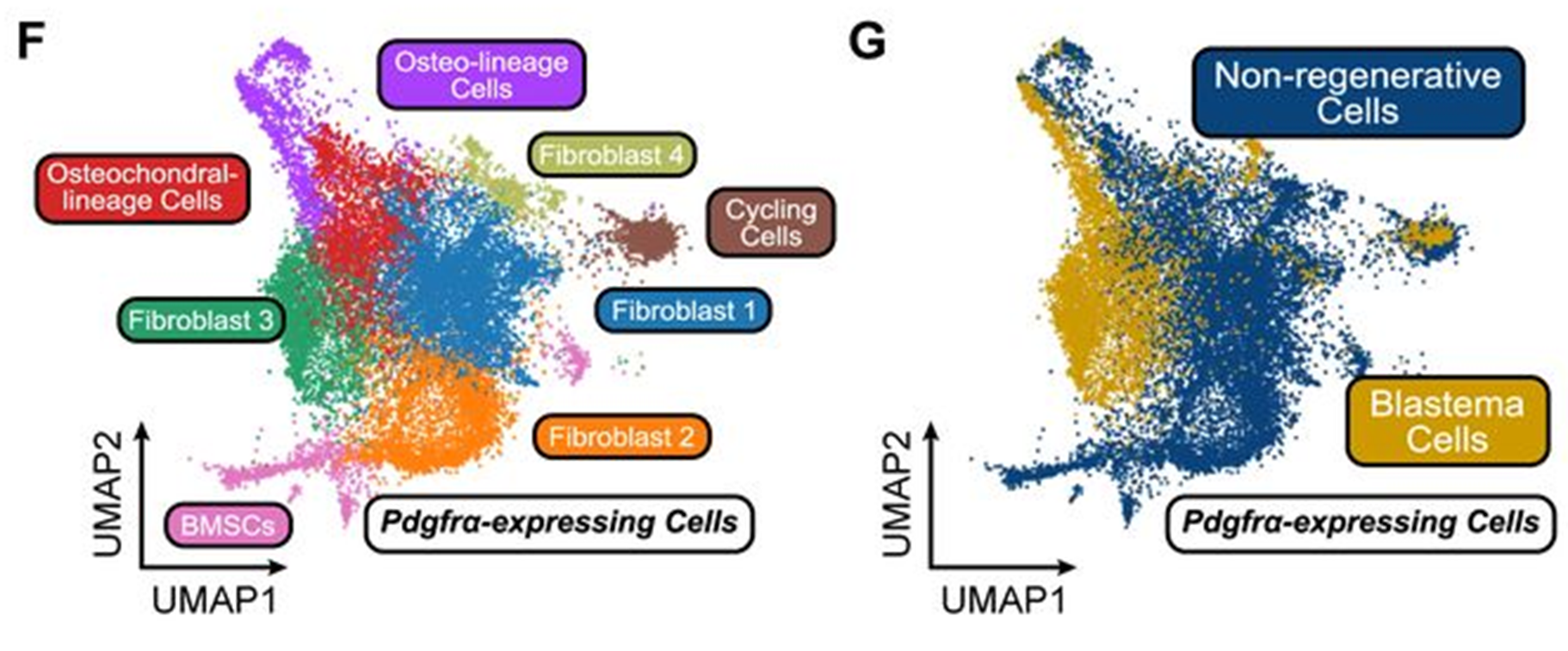
Figure 2: Uniform Manifold Approximation and Projection (UMAP) plots from figure 1 of Mui et al (2024) showing different fibroblast populations detected in single cell RNA sequencing of non-regenerative and regenerative (blastema) wounds. The same cells were coloured for the data set they originated from highlighting the prevalence of different fibroblast subtypes in the two wound conditions. Image made available under a CC-BY-NC-ND 4.0 International license.
A balance of hyaluronic acid and collagen determines wound stiffness and regenerative capability
4-methylumbelliferone (4-MU) is widely used to inhibit HA synthesis and is also an approved therapeutic (as hymecromone) in humans (Nagy et al., 2015). The team provided 4-MU in the mouse diet, followed by regenerative digit amputations. The 4-MU diet prevented digit regeneration as 4-MU digits were smaller in length and area, as well as bone volume, surface area and length, compared to control digits (Fig. 3). 4-MU digit wounds had more collagen and fibrotic ECM and were stiffer, more closely resembling a non-regenerative wound. There were also fewer OL fibroblasts, suggesting these are sensitive to the levels of HA. This led the team to propose a model where the balance of HA and collagen can determine the blastema/wound stiffness and fluid properties, with low stiffness and high fluidity leading to a regenerative blastema.
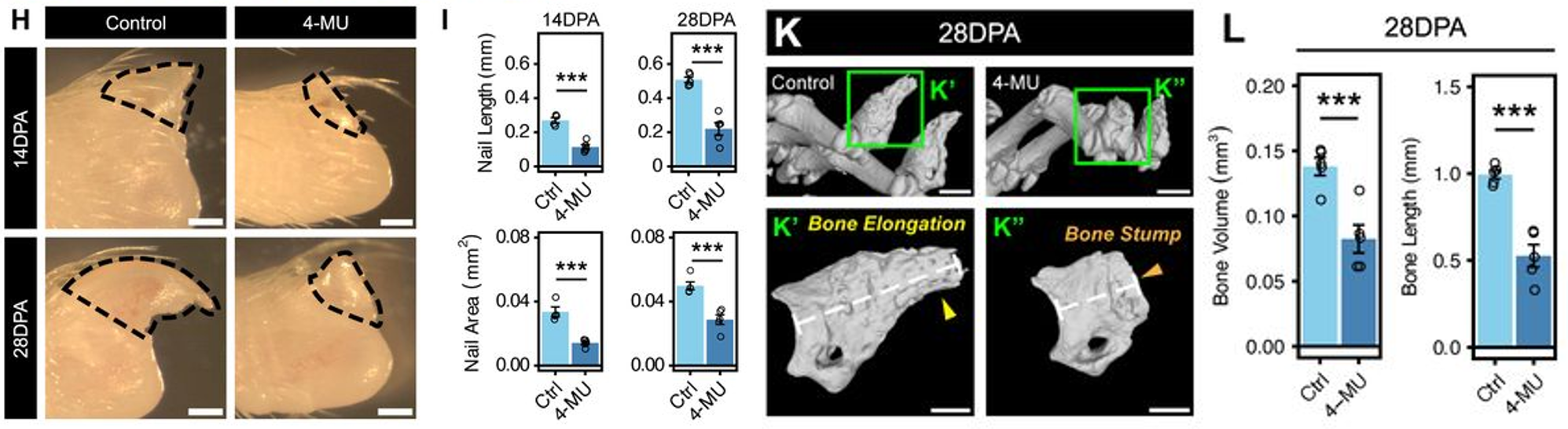
Figure 3: Gross images of control and 4-MU digits showed reduced nail length/area 14- and 28-days post amputation in 4-MU treated mice. Similarly, microcomputed tomography analysis of bones 28 days after amputation showed that bone volume and length was reduced in 4-MU treated mice. Adapted from figure 3 of Mui et al (2024). Image made available under a CC-BY-NC-ND 4.0 International license.
The defect in bone regeneration further inspired the team to look at the bone morphogenic protein (BMP) pathway. Immunofluorescence and Western blots were used to confirm that the effector of BMP signalling, pSmad1/5/8, was reduced in 4-MU digits compared to control, and that culturing fibroblasts on soft hydrogels led to the highest levels of pSmad1/5/8 (Fig. 4). This prompted the team to propose that BMP signalling can be affected by HA levels and that substrate stiffness can further tune the BMP signalling pathway.
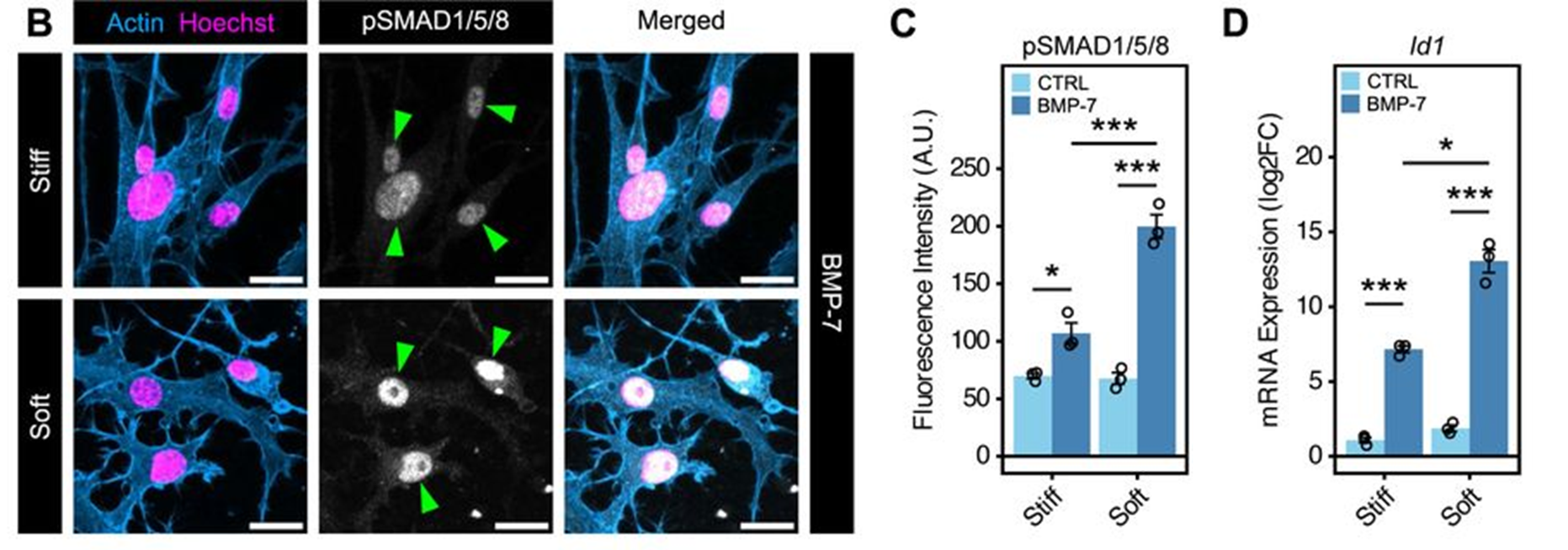
Figure 4: Immunofluorescent labelling of pSmad1/5/8 and qPCR analysis of its target gene Id1 from figure 5 of Mui et al (2024). Fibroblasts were cultured on soft or stiff hydrogels and treated with BMP-7. Increases in pSmad1/5/8 fluorescence and Id1 expression on the soft hydrogels showed BMP signalling can be tuned by substrate stiffness. Image made available under a CC-BY-NC-ND 4.0 International license.
Overexpressing HAPLN1 in stiff contexts can promote HA deposition and wound regeneration
Finally, the team asked if a soft substrate could promote the production of a regenerative ECM from fibroblasts. They first cultured fibroblasts on stiff and soft hydrogels and induced HA synthesis. Fibroblasts grown on soft gels were found to have increased expression of Hapln1 and had dense aggregates of HA and HAPLN1 together on the cell surface (Fig. 5). HAPLN1 and HA were also observed to colocalise together in uninjured digits, with less HAPLN1/HA in P2 (non-regenerative) and P3 (regenerative) regions. Therefore, HAPLN1 is mechanosensitive and able to retain or stabilise HA, suggesting soft substrates upregulate HAPLN1, which promotes HA retention, further promoting soft ECM and creating a positive feedback loop.
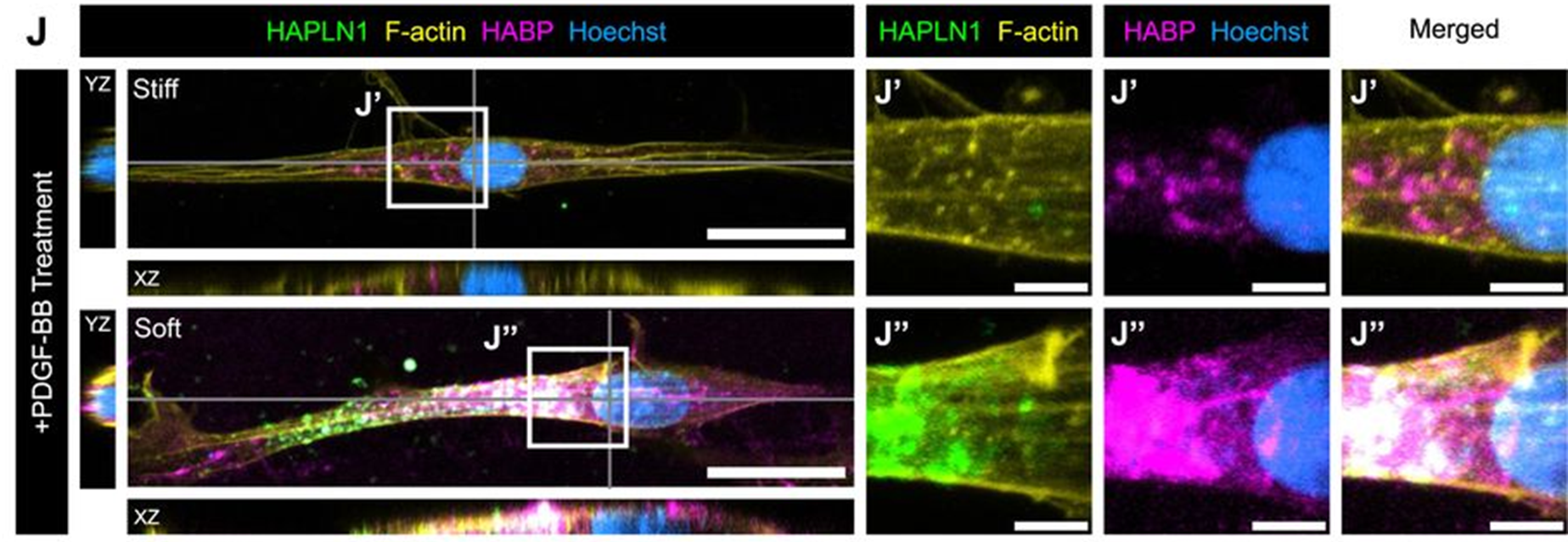
Figure 5: Immunofluorescence images of fibroblasts grown on stiff and soft hydrogels from figure 5 of Mui et al (2024). HA binding protein (HABP) and HAPLN1 levels increased in fibroblasts cultured on the soft substrate. Image made available under a CC-BY-NC-ND 4.0 International license.
To further test this, the team finished by overexpressing HAPLN1 (Hapln1OE) in fibroblasts. On stiff substrates Hapln1OE fibroblasts deposited more HA and reduced collagen fibrillogenesis compared to control cells. Furthermore, transplanting Hapln1OE fibroblasts into non-regenerative wounds (from immune-compromised mice, to avoid rejection) promoted HA accumulation, reduced fibrosis, enhanced bone repair and increased the presence of OL cells.
What I like about the preprint/why I think this new work is important
I was initially attracted to this preprint due to my interest in how mechanical cues affect cell responses and found it to be a tour de force in studying ECM composition and stiffness in wound healing. I really appreciated the combination of many different techniques to build a compelling narrative about the role of different cell types regulating HA networks in regenerative wounds and the influence stiffness has on this process. Finally, out of curiosity about the plethora of creams and gels in pharmacies promoting the properties of HA, I was interested to dig deeper into some primary literature and see what truth there is in the hype around HA.
Future directions and questions for the authors
- How is the initial regenerative or non-regenerative response to a wound determined? For example, are there differences in the stiffnesses or fibroblast populations of “prewounded” digits that determine the wound response?
- Can you comment further on what mechanisms individual cells use to transduce the mechanical cues from collagen/HA networks to transcriptional responses? Could these also be therapeutically targeted?
- How might infiltration of wound sites by cells from the immune system affect ECM remodelling? And vice versa?
References
- Aghigh, A., Bancelin, S., Rivard, M., Pinsard, M., Ibrahim, H., & Légaré, F. (2023). Second harmonic generation microscopy: a powerful tool for bio-imaging. Biophysical Reviews, 15(1), 43–70. https://doi.org/10.1007/s12551-022-01041-6.
- Chen, X., Nadiarynkh, O., Plotnikov, S., & Campagnola, P. J. (2012). Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nature Protocols, 7(4), 654–669. https://doi.org/10.1038/nprot.2012.009.
- Ganier, C., Rognoni, E., Goss, G., Lynch, M., & Watt, F. M. (2022). Fibroblast Heterogeneity in Healthy and Wounded Skin. Cold Spring Harbor Perspectives in Biology, a041238. https://doi.org/10.1101/cshperspect.a041238.
- Kuehlmann, B., Bonham, C. A., Zucal, I., Prantl, L., & Gurtner, G. C. (2020). Mechanotransduction in wound healing and fibrosis. Journal of Clinical Medicine, 9(5), 1–19. https://doi.org/10.3390/jcm9051423.
- Lendahl, U., Muhl, L., & Betsholtz, C. (2022). Identification, discrimination and heterogeneity of fibroblasts. Nature Communications, 13(1), 1–14. https://doi.org/10.1038/s41467-022-30633-9.
- Mast, B. A., Haynes, J. H., Krummel, T. M., Diegelmann, R. F., & Cohen, I. K. (1992). In vivo degradation of fetal wound hyaluronic acid results in increased fibroplasia, collagen deposition, and neovascularization. In Plastic and Reconstructive Surgery, (89)3, 503–509). https://doi.org/10.1097/00006534-199203000-00019.
- Moore, A. L., Marshall, C. D., Barnes, L. A., Murphy, M. P., Ransom, R. C., & Longaker, M. T. (2018). Scarless wound healing: Transitioning from fetal research to regenerative healing. Wiley Interdisciplinary Reviews: Developmental Biology, 7(2), 1–19. https://doi.org/10.1002/wdev.309.
- Mui, B. W. H., Wong, J. Y., Bray, T., Connolly, L., Wang, J. H., Winkel, A., Robey, P. G., Franze, K., Chalut, K. J., & Storer, M. A. (2024). Hyaluronic Acid and Emergent Tissue Mechanics Orchestrate Digit Tip Regeneration. BioRxiv. https://doi.org/10.1101/2024.12.04.626830.
- Nagy, N., Kuipers, H. F., Frymoyer, A. R., Ishak, H. D., Bollyky, J. B., Wight, T. N., & Bollyky, P. L. (2015). 4-Methylumbelliferone treatment and hyaluronan inhibition as a therapeutic strategy in inflammation, autoimmunity, and cancer. Frontiers in Immunology, 6(MAR), 1–11. https://doi.org/10.3389/fimmu.2015.00123.
- Rosińczuk, J., Taradaj, J., Dymarek, R., & Sopel, M. (2016). Mechanoregulation of wound healing and skin homeostasis. BioMed Research International, 2016. https://doi.org/10.1155/2016/3943481.
- Simkin, J., Sammarco, M. C., Dawson, L. A., Schanes, P. P., Yu, L., & Muneoka, K. (2015). The mammalian blastema: regeneration at our fingertips. Regeneration, 2(3), 93–105. https://doi.org/10.1002/reg2.36.
- Storer, M. A., & Miller, F. D. (2021). A finger on the pulse of regeneration: insights into the cellular mechanisms of adult digit tip regeneration. Current Opinion in Genetics and Development, 70, 1–6. https://doi.org/10.1016/j.gde.2021.04.002.
- Wynn, T. A., & Ramalingam, T. R. (2012). Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nature Medicine, 18(7), 1028–1040. https://doi.org/10.1038/nm.2807.
- Ziol, M., Handra-Luca, A., Kettaneh, A., Christidis, C., Mal, F., Kazemi, F., De Lédinghen, V., Marcellin, P., Dhumeaux, D., Trinchet, J. C., & Beaugrand, M. (2005). Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology, 41(1), 48–54. https://doi.org/10.1002/hep.20506.
doi: https://doi.org/10.1242/prelights.40001
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the biophysics category:
Active flows drive clustering and sorting of membrane components with differential affinity to dynamic actin cytoskeleton
Teodora Piskova
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
Mitochondrial Fatty Acid Oxidation is Stimulated by Red Light Irradiation
Rickson Ribeiro, Marcus Oliveira
Also in the developmental biology category:
Tissue mechanics and systemic signaling safeguard epithelial tissue against spindle misorientation
Ruoheng Li
Human pluripotent stem cell-derived macrophages modify development of human kidney organoids
Theodora Stougiannou
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
Also in the physiology category:
Trade-offs between surviving and thriving: A careful balance of physiological limitations and reproductive effort under thermal stress
Tshepiso Majelantle
Imaging cellular activity simultaneously across all organs of a vertebrate reveals body-wide circuits
Muhammed Sinan Malik
Wide-ranging behavioral dysfunction in two mouse models of pathological human variants in the GRIK2 kainate receptor gene
Pushpinder Singh
preLists in the biophysics category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
66th Biophysical Society Annual Meeting, 2022
Preprints presented at the 66th BPS Annual Meeting, Feb 19 - 23, 2022 (The below list is not exhaustive and the preprints are listed in no particular order.)
| List by | Soni Mohapatra |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
Biophysical Society Meeting 2020
Some preprints presented at the Biophysical Society Meeting 2020 in San Diego, USA.
| List by | Tessa Sinnige |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Biomolecular NMR
Preprints related to the application and development of biomolecular NMR spectroscopy
| List by | Reid Alderson |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
Also in the developmental biology category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
BSDB/GenSoc Spring Meeting 2024
A list of preprints highlighted at the British Society for Developmental Biology and Genetics Society joint Spring meeting 2024 at Warwick, UK.
| List by | Joyce Yu, Katherine Brown |
GfE/ DSDB meeting 2024
This preList highlights the preprints discussed at the 2024 joint German and Dutch developmental biology societies meeting that took place in March 2024 in Osnabrück, Germany.
| List by | Joyce Yu |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
2nd Conference of the Visegrád Group Society for Developmental Biology
Preprints from the 2nd Conference of the Visegrád Group Society for Developmental Biology (2-5 September, 2021, Szeged, Hungary)
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Society for Developmental Biology 79th Annual Meeting
Preprints at SDB 2020
| List by | Irepan Salvador-Martinez, Martin Estermann |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EDBC Alicante 2019
Preprints presented at the European Developmental Biology Congress (EDBC) in Alicante, October 23-26 2019.
| List by | Sergio Menchero et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |
Also in the physiology category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |











 (No Ratings Yet)
(No Ratings Yet)