Microtissue geometry and cell-generated forces drive patterning of liver progenitor cell differentiation in 3D
Posted on: 13 December 2020
Preprint posted on 28 October 2020
Article now published in Advanced Healthcare Materials at http://dx.doi.org/10.1002/adhm.202100223
Categories: biophysics, cell biology
Background
The differentiation and morphogenesis of progenitor cells into functioning tissues is governed by biochemical cues from neighbouring cells and other elements in the microenvironment. Moreover, it has been established that mechanical forces and mechanical signaling are pivotal for stem cell development and tissue behaviour. So far, much of the work relating stem cell behaviour to mechanical forces and signaling has been studied in 2D culture systems. However, the advent and more widespread use of 3D systems has proven to better replicate some in vivo conditions including increased cell-cell interactions and more freedom for motility and reorganization, which are key in differentiation and morphogenesis. Careful engineering is required to investigate cell responses in a 3D environment. Microwell-based platforms with different levels of complexity, have been widely implemented for various purposes including drug-screening, disease modeling and stem cell culture. However, implementation of 3D systems with mechanical constraints with tissue-specific contexts has been more limited. One such tissue-specific 3D models, is the liver, which in the embryo is derived from bipotential progenitor cells (hepatoblasts) that can give rise to hepatocytes or biliary epithelial cells, and eventually establish the liver lobes. It is known that the process of fate-specification, and eventual formation of the liver tissue and bile ducts, is spatially and temporally orchestrated by various cues. Additional factors including extracellular matrix stiffness, may also impact differentiation. To date, the relationship between mechanical signaling and liver progenitor fate specification in 3D has not yet been characterized. In their work, Berg et al (1) implemented an ECM scaffold-free hydrogel microwell-based method to produce arrays of liver progenitor cell microtissues, and characterized the 3D patterns of hepatocytic and biliary differentiation in various tissue geometries.
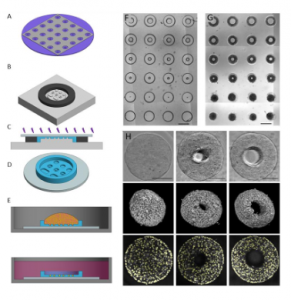
Key findings and developments
The authors generated a microwell-based approach to generate arrays of bipotential mouse embryonic liver (BMEL) cell 3D tissues with defined geometries. The baseline was a PEG substrate with 500µm side walls, with an insert array of microwells 200µm deep. This setup enables a wide variety of well geometries with high aspect ratio features, such as cylinder and toroid wells of varied inner and outer diameter. Moreover, the approach aims for tissues with approximately the same initial number of cells across geometries. As the microwells were non-fouling and non-adhesive, cells only adhere to themselves, and aggregated into dense 3D tissues constrained by the wells. In cylindrical wells, the cells aggregate and condense into a roughly cylindrical tissue. In toroid microtissues, the cells aggregate and condense away from the outer walls, but around the central post, resulting in a donut-shaped tissue. Image segmentation was used to locate individual nuclei to enable single cell analysis in 3D.
The authors then investigated hepatocytic and biliary phenotype patterning in cylindrical microtissues. After 72 hours of culture, the microtissues were fixed and immune-stained for biliary (OPN) and hepatocytic (HNF4a) markers. In the tissues, the cells expressing the biliary marker OPN were sparsely distributed throughout the tissue, while the cells expressing the hepatocytic marker HNF4a were found almost exclusively in the outer shell of the tissue. This was confirmed following the implementation of an image segmentation-based single cell analysis pipeline. Sorting cells into an inner, intermediate, and outer region based on shell coordinates, and calculating the percentage of cells positive for each marker, showed that there was a significantly different percentage of cells expressing the hepatocytic marker across all regions, with the outer region being the higher, and inner the lowest. Conversely, there was a significant increase in percentage of OPN+ cells in the intermediate region compared to the outer and inner regions.
The authors then investigated hepatocytic and biliary phenotype patterning in toroid microtissues. In these tissues, OPN positive cells were sparsely distributed across the 3D structure while HNF4a positive cells were found in the outer surface. Moreover, low levels of HNF4a positive cells were observed at the surface contacting the PEG pillar. Using two coordinates to sort cells into inner, intermediate, outer, and pillar regions showed that the percentage of cells positive for HNF4a was statistically significantly higher at the outer region compared to each other region, including the pillar. Percentage of OPN positive cells was lowest at the outer region, with a small increase of OPN positive cells at the pillar. Overall, untreated toroid microtissues had a statistically significant increase in percentage of OPN positive cells per tissue compared to cylinder tissues. Treatment with EGF increased biliary differentiation in both toroid and cylinder microtissues, however it did not disrupt the spatial patterning of HNF4a positive cells. EGF treatment also amplified the signal pattern of OPN expression, leading to OPN frequency increases in the intermediate area, in the region just outside the outer shell and near the pillar contacting region.
The authors went on to further characterize the architecture of the tissues, by staining the tissues for actin and E-cadherin along with HNF4a. E-cadherin was found to be expressed at low levels throughout, but was highest at the outer shell in both cylinder and toroid geometries. In toroid microtissues, the regions of increased E-cadherin expression correlated with the regions of increased HNF4a positive cells. This was highest at cell-cell junctions.
Next, the authors further explored the relationship between geometry and differentiation, by preparing oblong microtissues, with widths of 100, 200, or 300µm, and the length chosen such that the cross-sectional area is equal to that of a 400µm diameter circle. In 300µm-wide oblong wells, the spatial patterns of actin, E-cadherin and HN4a expression was like those observed in cylinder microtissues. Conversely, microtissues in the 200 and 100µm wide oblong wells condense such that they are pressed against the side walls, producing morphologies with flat sides and rounded caps. In the oblong tissues, dividing the tissues into core, cap and flat regions, showed that hepatocytic differentiation was excluded from flat regions.
Investigating the role of actin-myosin contractility on differentiation in 3D showed that disruption of actin-myosin contraction reduced biliary differentiation in all geometries, suggesting an important role of cell contractility for biliary differentiation. Although the level of hepatocytic differentiation was not affected, spatial patterning was. This latter phenotype was pronounced in toroid microtissues.
Finally, to further understand the mechanical behaviour of the microtissues, the authors implemented a 3D finite element method (FEM) based model, building from previously reported 2D and 3D tissue models. The model suggests that in the cylindrical microtissue, the outer shell region was primarily under tension, while the intermediate and core regions were entirely in compression, with the largest compressive stress in the intermediate region. In the toroid microtissue model, the same was true, however, the pillar contacting region was experiencing compression at comparable levels to the intermediate zone. The authors validated the model predictions by embedding microgels containing fluorescent beads into the microtissues, to measure forces within the tissue.
The results of the models suggest that geometry combined with the contractile shell drives regions of surface compression, which correlate with reduced hepatic differentiation and reduced E-cadherin expression. The authors explored this further by fabricating ‘double pillar’ and ‘dumbbell’ shaped microtissues. For these, the model predicted that the presence of pillars causes the tissues to contract away from the walls, eliminating any compressed surfaces. This behaviour was confirmed experimentally.
What I like about this preprint
I think the tools developed in this work are extremely valuable in the current aim of the scientific community to bridge our in vitro and in vivo knowledge on various topics, and to further reduce the need for animal experimentation. Further, I found this preprint exciting because of the plethora of methods used, and the interdisciplinary approach to the topic.
Open questions
- During the formation of the liver in the embryo, where does the vascular endothelium of the vessels supplying the hepatocytes come into play? Do these cells also influence differentiation?
- Is it know whether and how other stresses present in living organisms, such as sheer flow, influence differentiation?
- You investigated the various phenotypes in toroid and cylindrical microtissues., and later in the oblong microtissues and the double pillar and dumbbell-shaped microtissues. Can you expand further on why these different geometries were chosen and what aspects they reproduce of the organ?
- While your work focuses specifically on the liver, what would be your recommendation for studying microtissue geometry and cell-generated forces in 3D models of other organs?
- You mention in your discussion and conclusion that understanding tissue geometry and its effect on for instance, differentiation, could help us address developmental issues or pathologies such as those observed in cancer. Is it known whether and how tissue geometry changes in different pathological conditions and how this affects native cell forces, their differentiation and distribution?
References
- Berg IC et al, Microtissue geometry and cell-generated forces drive patterning of liver progenitor cell differentiation in 3D, bioRxiv, 2020.
doi: https://doi.org/10.1242/prelights.26383
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the biophysics category:
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
Mitochondrial Fatty Acid Oxidation is Stimulated by Red Light Irradiation
Rickson Ribeiro, Marcus Oliveira
ROCK2 inhibition has a dual role in reducing ECM remodelling and cell growth, while impairing migration and invasion
Sharvari Pitke
Also in the cell biology category:
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Taxane-Induced Conformational Changes in the Microtubule Lattice Activate GEF-H1-Dependent RhoA Signaling
Vibha SINGH
PIP5K-Ras bistability triggers plasma membrane symmetry breaking to define cellular polarity and regulate migration
Vibha SINGH
preLists in the biophysics category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
66th Biophysical Society Annual Meeting, 2022
Preprints presented at the 66th BPS Annual Meeting, Feb 19 - 23, 2022 (The below list is not exhaustive and the preprints are listed in no particular order.)
| List by | Soni Mohapatra |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
Biophysical Society Meeting 2020
Some preprints presented at the Biophysical Society Meeting 2020 in San Diego, USA.
| List by | Tessa Sinnige |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Biomolecular NMR
Preprints related to the application and development of biomolecular NMR spectroscopy
| List by | Reid Alderson |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
Also in the cell biology category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
December in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cell cycle and division 2) cell migration and cytoskeleton 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Matthew Davies et al. |
November in preprints – the CellBio edition
This is the first community-driven preList! A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. Categories include: 1) cancer cell biology 2) cell cycle and division 3) cell migration and cytoskeleton 4) cell organelles and organisation 5) cell signalling and mechanosensing 6) genetics/gene expression
| List by | Felipe Del Valle Batalla et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |











 (No Ratings Yet)
(No Ratings Yet)