A new calcium-activated dynein adaptor protein, CRACR2a, regulates clathrin-independent endocytic traffic in T cells
Posted on: 4 July 2018
Preprint posted on 22 June 2018
Article now published in Journal of Cell Biology at http://dx.doi.org/10.1083/jcb.201806097
A CRACR of a story… the identification of CRACR2a as a calcium responsive dynein adaptor involved in endocytosis at the T cell immune synapse.
Selected by Nicola StevensonCategories: cell biology, immunology
Background
Microtubule-based transport of organelles and molecular cargoes is conducted by two families of motor proteins, kinesins and dynein. In order to accommodate the vast structural diversity found within these cargoes, numerous forms of kinesin have evolved, each with different cargo binding capacities and motor properties. Dynein-1 on the other hand is solely responsible for all dynein-mediated cytoplasmic transport (dynein-2 is specific to intraflagellar transport). As such, dynein is reliant on adaptor proteins to couple cargo binding to motor activity in a more specific manner.
One of the key roles of dynein is to transport membrane-bound vesicles and organelles. These cargoes are recruited through interactions between the dynein adaptors and Rab GTPases, which reside in the membranes of specific organelles and help confer membrane identity. For example, the adaptor Bicaudal D can bind to Rab6 on Golgi membranes to mediate retrograde vesicle transport (1).
In this study Wang et al identify two new dynein adaptors, Rab45 and CRACR2a, which themselves possess Rab GTPase domains, showing for the first time a direct, functional interaction between a Rab GTPase and dynein.
Key findings
- GTPase domain containing proteins Rab45 and CRACR2a both bind to dynein-dynactin and activate processive motor activity in vitro and in cells
- The interaction between dynein and CRACR2a, but not dynein and Rab45, is calcium-dependent
- CRACR2a puncta appear at the immune synapse and cluster at the microtubule organising centre (MTOC) upon T cell activation-induced elevation of intracellular calcium in Jurkat T cells
- These puncta slowly comigrate with actin before recruiting dynein and rapidly travelling to the MTOC suggesting a coordinated handover of cargo from the cortex to microtubules (Figure 1)
- CRACR2a puncta represent endocytic vesicles, formed in the absence of clathrin function
- The transmembrane protein CD47 is found in the vesicles carried by dynein-CRACR2a
CONCLUSION: CRACR2a is involved in a new type of calcium-stimulated, clathrin-independent endocytic pathway at the T cell synapse and CD47 is one of its endocytic cargoes.
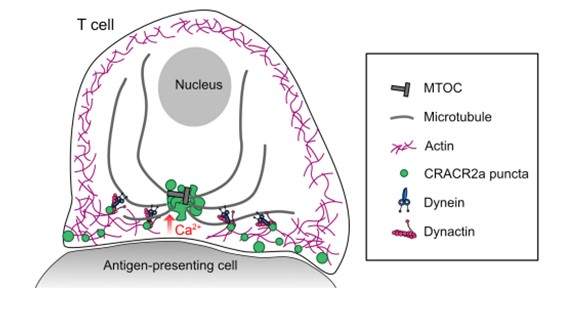 Figure 1. Schematic of CRACR2a-dynein mediated transport in T cells;
Figure 1. Schematic of CRACR2a-dynein mediated transport in T cells;
from Fig. 6. of the preprint
Importance
I chose to highlight this paper because, whilst the identification of new dynein adaptors is always noteworthy, the identification of a single adaptor which not only specifies a vesicle for transport, but also the environmental conditions under which transport should proceed is particularly exciting. Such tight links between signalling and transport using so few proteins is unusual and perhaps necessary for a rapid response. The role of this motor-adaptor complex in clathrin-independent endocytosis (CIE) is also of vital importance to our understanding of this pathway. The molecular mechanisms underpinning CIE remain elusive and, whilst it is not yet obvious how soon CRACR2a is recruited in this process, this discovery could potentially provide an anchor point from which to puzzle outwards. Finally, the observed recruitment of CRACR2a-dynein to endocytic vesicles is a step towards understanding how the enigmatic hand-over of vesicles between the actin and microtubule networks is coordinated at the cell cortex.
Future questions
- How important is the Rab GTPase domain and does this bind to other effectors?
- What other calcium signalling events activate CRACR2a-dynein activity? This study extensively explores the role of CRACR2a in CIE upon T cell activation but is it active during other signalling events such as neurotransmission? And does it always bind to the same cargo if the source of the stimulus changes?
- What is the link between calcium signalling and the type of cargoes that are internalised by CRACR2a? If CRACR2a mediated trafficking is responsive to calcium signalling then it is likely that targeted cargoes have some relevance to the physiological situation the cells are responding too, for example immune synapse organisation.
- How essential is CRACR2a to immune synapse function?
References
- Matanis et al. Bicaudal-D regulates COPI-independent Golgi-ER transport by recruiting the dynein-dynactin motor complex. Nat Cell Bio 4(12):986-92
Sign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Cell cycle-dependent mRNA localization in P-bodies
Mohammed JALLOH
Control of Inflammatory Response by Tissue Microenvironment
Roberto Amadio
Notch3 is a genetic modifier of NODAL signalling for patterning asymmetry during mouse heart looping
Bhaval Parmar
Also in the immunology category:
Control of Inflammatory Response by Tissue Microenvironment
Roberto Amadio
G6b-B antibody-based cis-acting platelet receptor inhibitors (CAPRIs) as a new family of anti-thrombotic therapeutics
Simon Cleary
Feedback loop regulation between viperin and viral hemorrhagic septicemia virus through competing protein degradation pathways
UofA IMB565 et al.
preLists in the cell biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |
Also in the immunology category:
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |











 (No Ratings Yet)
(No Ratings Yet)