Osmolarity-independent electrical cues guide rapid response to injury in zebrafish epidermis
Preprint posted on 5 August 2020 https://www.biorxiv.org/content/10.1101/2020.08.05.237792v1
Article now published in eLife at http://dx.doi.org/10.7554/elife.62386
Charging the cellular migration- dissecting electrical cues that guide the wound response!
Selected by Ankita JhaCategories: cell biology
Context-
Epithelial tissue can act as a strong resistance barrier by minimizing the passive flow of ions down the concentration gradients that maintain trans-epithelial potential (TEP). This can generate a significant electrical field around the tissue. Wounding or injury in the epithelial tissue can cause electric flow currents in the direction of the injury, which can be maintained for a long time. This regulates the wound response by guiding cells to migrate and cover the wound (Reid and Zhao, 2014). Cell migration in response to the electrical field has been hypothesized to be controlled by actin alignment, reorganization, and distribution of plasma membrane proteins and organelles (Robinson KR. 1985). But what are the electrical cues in-vivo that guide cell polarization and migration during a wound response? In this preprint, the authors test the role of osmolarity, tonicity and the electrical field in the wound response.
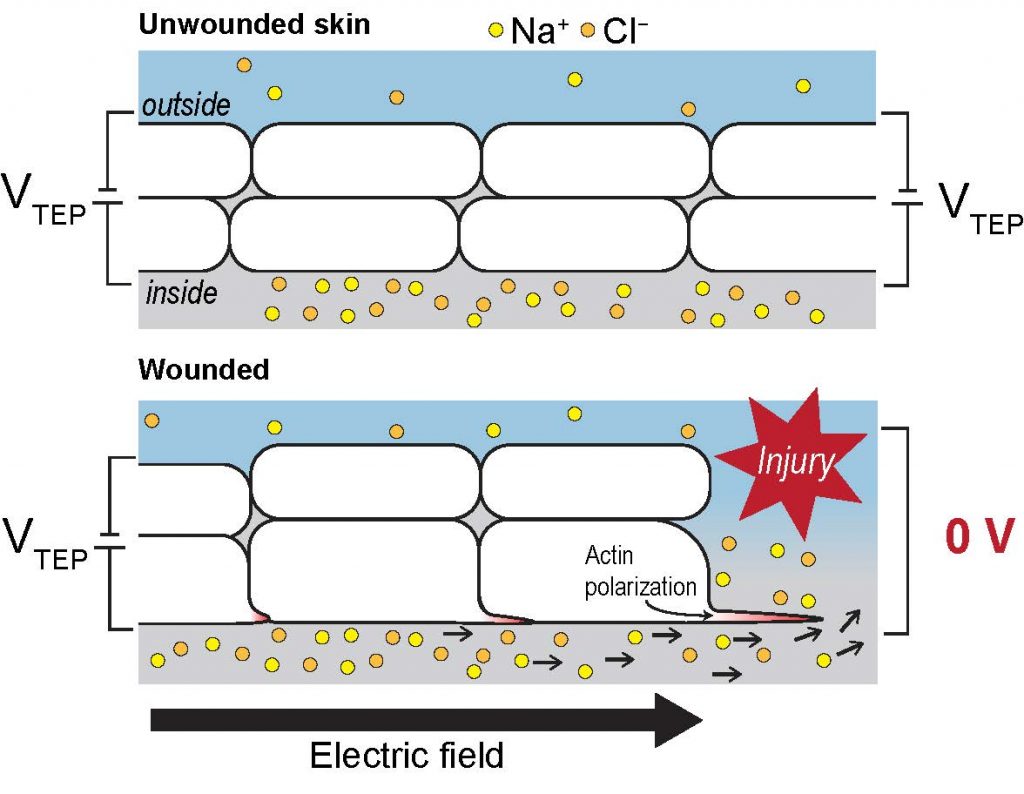
Major findings-
- Tissue laceration in zebrafish tail leads to tissue contraction and induces a migratory response which is concomitant with calcium spikes at different rates in the first few minutes of the injury.
- Injury leads to actin polarization with the formation of actin-rich ruffles different from isolated keratocytes.
- This polarization was evident up to several hundred micrometers away from the laceration.
- Osmolarity- Cell speed was strongly reduced in sodium chloride solution compared to other isosmotic solutions, which suggested that wound-induced cell migration depends on the local sodium-chloride ionic activity, not just the osmotic effect. Cells largely lacked polarization and actin reorganization in sodium chloride isosmotic solution.
- Tonicity-Authors show that cell migration and wound response is independent of the tonicity of the isosmotic solutions (identical concentration of different salts inducing water flow).
- Electrical field- One of the cues during injury is the disruption of trans-epithelial potential, which is maintained by sodium and chloride ions across the skin. Authors show that cellular wound response is directional towards the cathode and the cells respond in a polarized fashion towards exogenous wound cues.
What I like about the preprint-
The idea that cells respond to differences in the local electrical field around tissues have been put forward some time ago, but a careful dissection of mechanisms has yet not been achieved. This work focusses on carefully dissecting different hypotheses that would be responsible for polarized cell migration after injury in zebrafish. This work also reinforces the idea that trans-epithelial potential and generation of electrical flows is an important factor to be considered in wound response in-vivo.
Questions to the authors-
- This work shows that trans-epithelial potential (TEP) is important and disruption of TEP can induce cell polarization. It has been suggested that TEP is maintained by ion channels and sodium-potassium pumps. With wounding, electrical flows are generated locally eliciting cellular responses. Could the authors suggest how are these flows maintained over time?
- It has also been shown that cell polarization is evident several hundred microns away from the wound. How is this response elicited when the changes in the electrical flows are so local? Or can small lacerations cause large scale electrical flows?
References-
Robinson KR. The responses of cells to electrical fields: a review. J Cell Biol. 1985;101(6):2023-2027. doi:10.1083/jcb.101.6.2023
Reid B, Zhao M. The Electrical Response to Injury: Molecular Mechanisms and Wound Healing. Adv Wound Care (New Rochelle). 2014;3(2):184-201. doi:10.1089/wound.2013.0442
Posted on: 28 October 2020
doi: https://doi.org/10.1242/prelights.25498
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Fetal brain response to maternal inflammation requires microglia
Alteration of long and short-term hematopoietic stem cell ratio causes myeloid-biased hematopoiesis
Clusters of lineage-specific genes are anchored by ZNF274 in repressive perinucleolar compartments
preLists in the cell biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |











 (No Ratings Yet)
(No Ratings Yet)