Microtubule-independent movement of the fission yeast nucleus
Posted on: 6 October 2020
Preprint posted on 30 August 2020
Article now published in Journal of Cell Science at http://dx.doi.org/10.1242/jcs.253021
Can the nucleus of a cell move in the absence of dynamic microtubules? Ashraf et al. uncover microtubule-independent nuclear movement in fission yeast cells.
Selected by Leeba Ann ChackoCategories: cell biology, microbiology
Background:
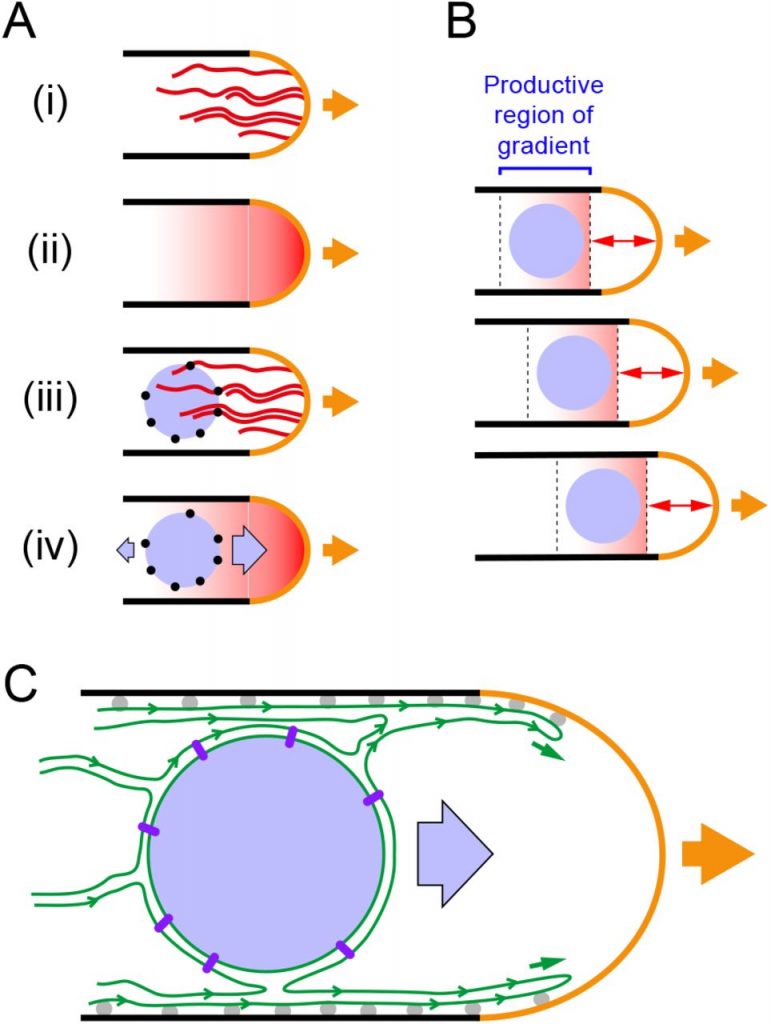
The fission yeast is a rod-shaped unicellular eukaryote that divides symmetrically to produce two similar-sized daughter cells. The cells initially grow in a unidirectional manner through polarized tip extension until a length of about 9.0-9.5 microns, then they begin to grow in a bi-directional manner until a length of ~14 μm after which they divide [1].
During monopolar cell growth, the nucleus is dynamically positioned at the geometric center of the cell through the polymerization-based pushing forces of anti-parallel microtubule bundles that emanate from the microtubule organizing center (MTOC) to the poles of the cell [2-5]. The position of the nucleus dictates the localization of the anillin-like protein, Mid1p, which recruits a series of proteins that aid the assembly of the actomyosin ring for cell division [6]. Tubulin mutants show nuclear-positioning defects, thus affecting the site of cell division and inheritance of cellular components post-mitosis [4].
While the importance of microtubule dynamics in positioning the nucleus has been established, it is not known if the nucleus can move in the absence of microtubules. Ashraf et al. show that nuclear movement persists in a unidirectional manner in the absence of microtubules and this movement depends on actin cables that originate from the growing tip of the cell as well as the organization of the endoplasmic reticulum (ER).
Key findings:
Monopolar–growing cells require the action of active forces to enable the movement of the nucleus in the direction of the growing cell tip to maintain the position of the nucleus at the gyrometric center of the cell. These forces were thought to depend on the microtubule bundle pushing forces. However, Ashraf et al. showed that even after depolymerizing microtubules using a drug, the nucleus moved at velocities like control cells in the direction of the growing tip thus staying mostly at the geometric center of the cell. The authors refer to this movement as “Microtubule-independent nuclear movement (MINM)”.
To understand the mechanism behind MINM, the authors looked at actin cables because they extend from the growing tip of the cell towards the inside of the cell and can encounter the nucleus. The authors found that in cells devoid of the actin-nucleator formin 3 (for3), or cells with a single point mutation in the actin-binding region of the Formin Homology 2 domain, the nucleus remained stationary upon depolymerizing microtubules indicating that actin cables are necessary for MINM to occur.
To check if the physical link between the actin cables and the nucleus is responsible for MINM, the authors compared nuclear movement close to and away from the growing tip by depolymerizing the microtubules and then centrifuging the cells. The nuclei that were displaced away from the growing tip upon centrifugation showed no movement while those that were displaced towards the growing tip continued to move towards it indicating that MINM can occur only if the nucleus is close to actin cables at the growing tip.
Since class V myosins are involved in actin filament organization, the authors set out to see if these myosins contribute to MINM. While myo51Δ and myoVΔ nuclei showed movement towards the growing tip upon microtubule depolymerization, myo52Δ nuclear movement was more complex wherein a proportion of the cells displayed either no nuclear movement and/or sudden movements which includes movement away from the growing tip. The authors attribute this aberrant nuclear movement observed in microtubule-depolymerized myo52Δ cells to the ability of myo52 to affect actin cable organization.
As the nuclear envelope and cortex in S. pombe cells are enveloped by the ER, the authors hypothesized that the actin cables may be causing MINM through the ER. To test this, the authors observed nuclear movement in cells devoid of the ER-localized transmembrane proteins, which shows large scale detachment of the ER from the plasma membrane. Interestingly, they found that most of these cells showed no nuclear movement upon microtubule depolymerization indicating that these ER proteins work along with actin cables to enable MINM.
Lastly, to test whether MINM occurs in the presence of microtubules, the authors looked at nuclear movement in cells devoid of for3 as well as cells devoid of the ER-localized transmembrane proteins. Interestingly, the nucleus in cells without for3 showed increased fluctuations compared to wild-type cells indicating that the presence of actin cables helps dampen the MT-based pushing forces. However, deleting ER-localized transmembrane proteins did not show the same effect and the authors speculate that this is possibly due to the collapse of the ER into the cytoplasm dampens the MT-pushing movement.
What I liked about this preprint:
The preprint was very well written and easy to follow. This is the first time I am coming across literature that shows the role of actin cables in moving the nucleus in fission yeast. As soon as I saw the title of this preprint, I was interested! Even though MINM is slow, it influences how the cell divides and that is remarkable to me.
Questions for the authors:
- Figure 1I shows that in the absence of microtubules, there is no relationship between nuclear velocity and the position of the nucleus. However, Figure 4 shows that in the absence of microtubules, the nuclear velocity is greatest when the nucleus is displaced towards the growing tip and least when it was displaced away from the growing tip. Why do you suppose there is no correlation between the position of the nucleus and nuclear velocity in the first experiment but there is a correlation in the second experiment?
- Previous studies have shown that when microtubules are depolymerized in cells under specific conditions, over time the cell begins to display polarity defects [7]. Additionally, cells with shorter microtubules also show polarity defects [4]. Would it be worthwhile to see in which direction the nucleus moves when there are multiple growing tips in a cell?
- It is surprising that despite changes in actin cable organization in both myo52Δ and myoVΔ cells, only myo52Δ nuclei show complex movement. Could there be something more at play besides subtle differences in the actin organization between myo52Δ and myoVΔ cells?
- You have shown that increased nuclear fluctuations in for3Δ mutants could be causing the observed increased frequency of misplaced septa. However, the nuclear fluctuations are unaltered in scsΔ cells and yet there is an increased frequency of misplaced septa in these mutants. If the nuclear dynamics are unaffected in the scsΔ mutants, how are a significant proportion of these cells dividing asymmetrically?
- You have shown that the microtubule bundle numbers are unchanged in for3Δ mutants. However, could changes in microtubule orientation [8] or changes in microtubule dynamics (growth rates, shrinkage rates, catastrophe frequencies and dwell times) contribute to the increased nuclear fluctuations?
References:
- Hercyk, B.S., et al., A novel interplay between GEFs orchestrates Cdc42 activity during cell polarity and cytokinesis in fission yeast. J Cell Sci, 2019. 132(23).
- Hagan, I. and M. Yanagida, Evidence for cell cycle-specific, spindle pole body-mediated, nuclear positioning in the fission yeast Schizosaccharomyces pombe. J Cell Sci, 1997. 110 ( Pt 16): p. 1851-66.
- Tran, P.T., et al., A mechanism for nuclear positioning in fission yeast based on microtubule pushing. J Cell Biol, 2001. 153(2): p. 397-411.
- Sawin, K.E. and H.A. Snaith, Role of microtubules and tea1p in establishment and maintenance of fission yeast cell polarity. J Cell Sci, 2004. 117(Pt 5): p. 689-700.
- Daga, R.R., A. Yonetani, and F. Chang, Asymmetric microtubule pushing forces in nuclear centering. Curr Biol, 2006. 16(15): p. 1544-50.
- Paoletti, A. and F. Chang, Analysis of mid1p, a protein required for placement of the cell division site, reveals a link between the nucleus and the cell surface in fission yeast. Mol Biol Cell, 2000. 11(8): p. 2757-73.
- Sawin, K.E. and P. Nurse, Regulation of cell polarity by microtubules in fission yeast. J Cell Biol, 1998. 142(2): p. 457-71.
- Feierbach, B. and F. Chang, Roles of the fission yeast formin for3p in cell polarity, actin cable formation and symmetric cell division. Curr Biol, 2001. 11(21): p. 1656-65.
doi: https://doi.org/10.1242/prelights.25059
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Taxane-Induced Conformational Changes in the Microtubule Lattice Activate GEF-H1-Dependent RhoA Signaling
Vibha SINGH
PIP5K-Ras bistability triggers plasma membrane symmetry breaking to define cellular polarity and regulate migration
Vibha SINGH
Also in the microbiology category:
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Microbial Feast or Famine: dietary carbohydrate composition and gut microbiota metabolic function
Jasmine Talevi
Citrobacter rodentium infection activates colonic lamina propria group 2 innate lymphoid cells
André Luiz Amorim Costa, Marcus Oliveira
preLists in the cell biology category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
December in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cell cycle and division 2) cell migration and cytoskeleton 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Matthew Davies et al. |
November in preprints – the CellBio edition
This is the first community-driven preList! A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. Categories include: 1) cancer cell biology 2) cell cycle and division 3) cell migration and cytoskeleton 4) cell organelles and organisation 5) cell signalling and mechanosensing 6) genetics/gene expression
| List by | Felipe Del Valle Batalla et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |
Also in the microbiology category:
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)