SorCS1-mediated Sorting of Neurexin in Dendrites Maintains Presynaptic Function
Posted on: 27 February 2019
Preprint posted on 17 February 2019
Article now published in PLOS Biology at http://dx.doi.org/10.1371/journal.pbio.3000466
What mechanisms instruct axon-dendritic polarity of neurons? The dendritic sorting receptor SorCS1 maintains cell compartment-specific protein composition.
Selected by Carmen AdriaensCategories: cell biology, neuroscience
Background
Neurons are polarized cells with two distinct compartments: dendrites and axons. These contain the post-and pre-synaptic specialization, respectively, which vary greatly in protein composition. Proper neuronal development, neuronal function and neuronal plasticity depend on the precise targeting of proteins to the right compartment. The bulk of protein synthesis in neurons occurs at the cell body. Thus, a central conundrum in Neuroscience remains: how are axonal and dendritic proteins differentially located to distinct compartments within the same cell?
Neurons have developed different mechanisms to ensure proper axonal protein targeting1. For instance, when protein subcellular distribution initially happens in a non-discriminatory fashion, protein cargo can be removed exclusively from the dendritic surface through a mechanism called selective endocytosis/retention2. As a result, protein cargo becomes enriched at the axonal surface. Alternatively, axonal cargo can be first inserted into the somatodendritic plasma membrane, and only then internalized from the cell surface and rerouted via endosomally derived carriers to the axon through a mechanism called transcytosis2.
In this preprint, Ribeiro et al. examined the role of the sorting receptor SorCS1 in the transcytosis-mediated axonal targeting of Neurexin (Nrxn), a presynaptic cell adhesion molecule essential for synaptogenesis and neuronal transmission3. SorCS1 belongs to the family of VPS10P-domain sorting receptors4, which are prominently expressed in the brain. They have recently emerged as key regulators of intracellular trafficking of synaptic receptors5–7 , but the mechanism through which they do this is yet uncharacterized. Here, Ribeiro and colleagues elucidate part of the cellular and molecular mechanisms that SorCS proteins use to regulate the trafficking of synaptic receptors.
Key Findings
First, the authors show that the axonal surface of mature cortical primary neurons displays twice as much endogenous Nrxn1a as the dendritic surface, indicating polarization towards the axon (see figure below). However, when the cells are permeabilized, Nrxn1a is mostly detected in dendrites. Permeabilization makes it possible to detect also intracellular proteins, indicating that Nrxn1a is trafficked to both compartments. To understand the mechanism responsible for axonal polarization of Nrxn1α the authors performed live-cell imaging experiments to monitor the transport of Nrxn1a throughout the secretory pathway. They showed that Nrxn1a is first trafficked to dendrites, appearing in the axon only after a long delay. Thus, they hypothesized that newly synthesized Nrxn1a is first trafficked to the somatodendritic compartment, where it is inserted into the plasma membrane. Indeed, when they monitored endocytosis, Nrxn1a was internalized from this membrane and further transcytosed, through the cell, from the dendritic surface to the axonal compartment.
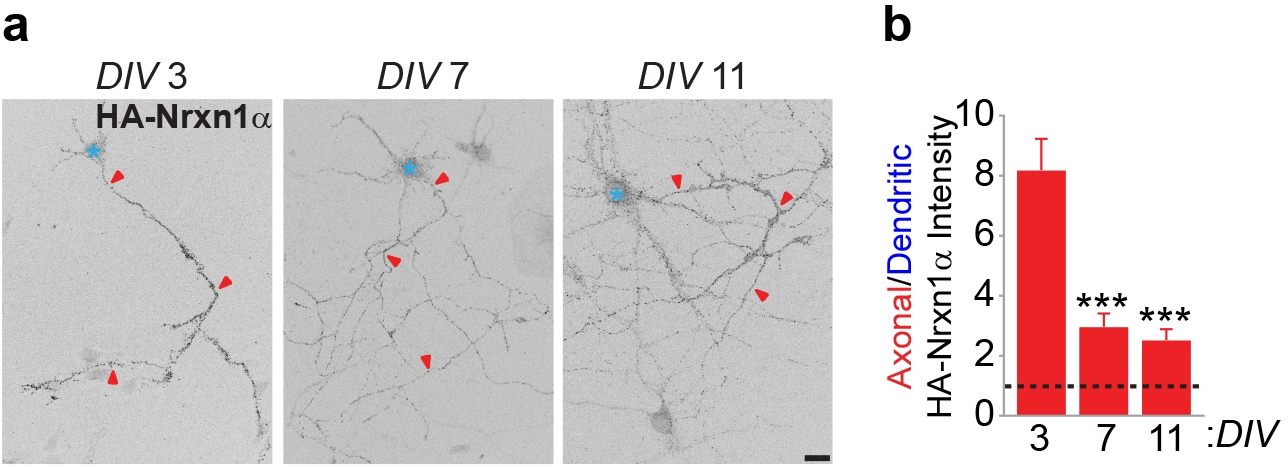
Figure. (a) Surface endogenous Nrxn1a is axonally polarized in mouse cortical neurons. Red arrowheads indicate the axon and blue asterisks mark the cell body. (b) ratio of the axonal/dendritic HA intensity. DIV, days in vitro. Figure adapted from the preprint figure 3 by Ribeiro et al. made available under a CC-BY-NC-ND 4.0 International license.
One factor that could be involved in this process is SorCS1, an endosomal sorting receptor that cycles between the plasma membrane and the endosomal pathway4. Indeed, as previous work had shown that SorCS1 directly binds Nrxn in cis7, the authors tested whether this protein was required for Nrxn1α transcytosis. Loss of SorCS1 resulted in an accumulation of Nrxn1a on the dendritic surface and its depletion from the axonal surface, shifting Nrxn1α surface polarization from axonal to dendritic. This polarization defect was due to an impaired transition of internalized Nrxn1a from early endosomes (EEs) to recycling endosomes (REs) in the somatodendritic compartment. Normally, upon internalization from the dendritic plasma membrane, cargo proteins are incorporated into small carrier vesicles that fuse with existing EEs. From EEs, cargo is sorted to REs. When this transition is affected (like in the case of SorCS1 loss), cargo accumulates in EE, and is either degraded via lysosomes or recycled back to the dendritic membrane.
To gain more mechanistic insight into how SorCS1 functions, the authors set out to find factors that interact with this protein. They identified the endosomal associated protein, Rip11, as a new SorCS1 binding partner. Rip11 has been previously involved in sorting of cargo from early to recycling endosomes in non-neuronal cells, and here they showed that it is also required to maintain axon-dendritic polarity of Nrxn1a.
With these findings in mind, Ribeiro et al. proposed that SorCS1 and Rip11 form a protein complex that localizes to dendritic endosomes and sorts internalized Nrxn1α from early to recycling endosomes. This could explain the increase in surface dendritic levels of Nrxn1α and the concomitant decrease in surface axonal levels observed in Sorcs1 KO cells.
Finally, the authors tested the impact of genetic ablation of SorCS1, which decreases the axonal surface targeting of Nrxn1a, on Nrxn-dependent neuronal transmission and synaptogenesis. Indeed, depletion of SorCS1 compromised Nrxn-dependent synapse assembly and neuronal transmission. In summary, these findings expand the list of neuronal cargoes that undergo transcytosis, which so far is very limited2, and support the notion that SorCS1-mediated sorting in dendrites is required to maintain axon-dendritic polarity and normal neuronal function.
What do I think about this preprint / Open Questions?
I like this preprint a lot! It is a detailed study, and carefully performed. One experiment I particularly like, and that I think should become more common in cell biology research, is the endogenous tagging of proteins using CRISPR/Cas9. For instance, with this technique, the authors showed that Nrxn1α does not only localize to axons, but could also be found (albeit potentially temporarily) in the dendrites. As such, the authors could overcome the lack of good antibodies, and used these observations as the basis for their study. In addition, the live-cell imaging experiments in this work are very impressive. I thought the videos of cargo trafficking from one cellular compartment to the other in real life were awesome!
However, these observations also raise some questions that, in the future, may need further clarification. For instance, what is the biological function of the dendritic pool of Nrxn? Is dendritic Nrxn merely a reservoir of readily-synthesized protein to be delivered to the axonal membrane, or does dendritically localized Nrxn have a function in the dendritic compartment? As dendritic/postsynaptically expressed Nrxn has been shown to inhibit synapse formation8, it would be interesting to further study other possible functions of dendritic Nrxn, particularly of intracellular Nrxn, which is abundantly present in dendrites.
One important finding is that the axonal-dendritic balance of Nrxn is regulated by neuronal activity. Does this mean that SorCS1-mediated sorting can dynamically regulate Nrxn polarity? How do neuronal activity and SorCS1-mediated sorting of Nrxn crosstalk in order to ensure proper protein distribution and neuronal functioning? And further, how much can these Nrxn-specific observations be generalized to explain neuronal activity-dependent compartment diversification?
In summary, I think this study provides important insights into the mechanisms controlling the intracellular trafficking of a compartmentally polarized protein, which helps to better understand subcellular protein distribution in polarized cells. With the toolbox the authors developed for this study, I am convinced many more questions can be answered, and thus I look forward to read the follow up stories, too!
References
- Bentley, M. & Banker, G. Nat. Rev. Neurosci. 17, 611–622 (2016).
- Winckler, B. & Mellman, I. Cold Spring Harb. Perspect. Biol. 2, a001826–a001826 (2010).
- Südhof, T.C. Cell 171, 745–769 (2017).
- Willnow, T.E., Petersen, C.M. & Nykjaer, A. Nat. Rev. Neurosci. 9, 899–909 (2008).
- Ma, Q. et al. JCI Insight 2, (2017).
- Glerup, S. et al. Mol. Psychiatry 21, 1740–1751 (2016).
- Savas, J.N. et al. Neuron 87, 764–780 (2015).
- Taniguchi, H. et al. J. Neurosci. 27, 2815–2824 (2007).
doi: https://doi.org/10.1242/prelights.8991
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Cell cycle-dependent mRNA localization in P-bodies
Mohammed JALLOH
Control of Inflammatory Response by Tissue Microenvironment
Roberto Amadio
Notch3 is a genetic modifier of NODAL signalling for patterning asymmetry during mouse heart looping
Bhaval Parmar
Also in the neuroscience category:
Sexually dimorphic role of diet and stress on behavior, energy metabolism, and the ventromedial hypothalamus
Jimeng Li
Enhancer-driven cell type comparison reveals similarities between the mammalian and bird pallium
Rodrigo Senovilla-Ganzo
Autism gene variants disrupt enteric neuron migration and cause gastrointestinal dysmotility
Rachel Mckeown
preLists in the cell biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |
Also in the neuroscience category:
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |











 (No Ratings Yet)
(No Ratings Yet)