Transcriptome analysis of Plasmodium berghei during exo-erythrocytic development
Posted on: 2 May 2019
Preprint posted on 7 February 2019
How is the Plasmodium parasite’s progeny different in the liver and the blood? A transcriptomic study provides clues and implications for pathology.
Selected by Mariana De NizCategories: bioinformatics, cell biology, genetics, microbiology
Transcriptome analysis of Plasmodium berghei during exo-erythrocytic development
Background
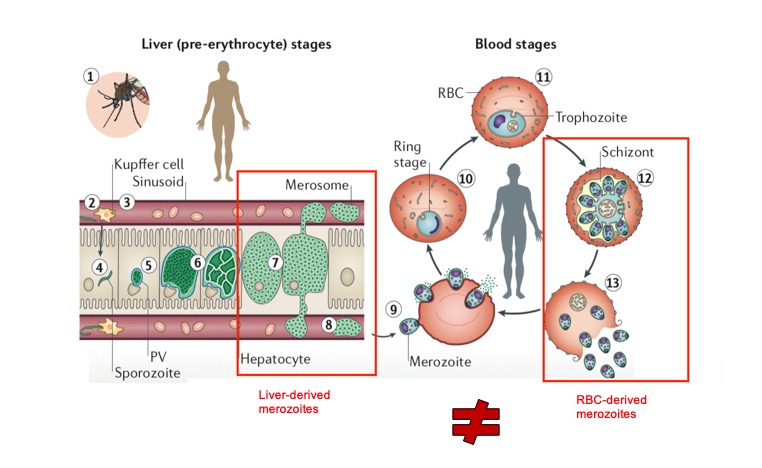
The Plasmodium parasite, causative of malaria, has two key stages of development in the mammalian host: a pre-erythrocytic stage (also known as liver stage), and an erythrocytic stage which involves continuous invasion of red blood cells, and is responsible for pathologies that make malaria a heavy burden for global health efforts. Only the blood stages are clinically symptomatic, resulting in malaria and its syndromes and complications. Vast research effort has been devoted over many decades to understanding erythrocytic stages of Plasmodiumdevelopment, in an attempt to find an ‘Achilles heel’ on the parasite, to render it susceptible to anti-malarial treatment and vaccines.
Pre-erythrocytic stages are a silent stage of parasite development, yet massive parasite asexual replication occurs, whereby a single parasite can produce thousands of progeny in a short amount of time, making this one of the fastest replication rates among eukaryotes. The parasite’s developmental cycle involves replication by schizogony, during which nuclear division, and organelle growth and replication, occur (Figure 1) (1). At the end of the process, segregation occurs ensuring that each progeny parasite has a set of functional organelles. The erythrocytic stages of development also involve asexual replication by schizogony and also culminate in the production of merozoites. Whether schizogony at both stages is regulated in a similar manner, is unknown.
In their work, Caldelari et al performed a genome-wide RNA-seq analysis at various temporal sub-stages of Plasmodium berghei throughout the parasite’s development in the liver. The main aim of the work was to better understand gene regulation and metabolic networks during pre-erythrocytic stage schizogony, and to determine whether differences exist between pre-erythrocytic and erythrocytic stage-derived merozoites and/or schizonts (2).
Key findings
- The transcription profiles of merozoites and schizonts arising from liver stages differ substantially from those of merozoites and schizonts arising from blood stages.
- A main difference in blood and liver stage parasites was found to be in metabolic processes, due to the high usage of fatty acids to generate various parasite membranes in pre-erythrocytic stages.
- A significant difference existed in genes related to mechanisms of egress from the respective host cells.
- Four genes particularly upregulated in blood stage schizonts were related to immune evasion, antigenic variation, and sialic-acid-dependent invasion of red blood cells. Of particular relevance is the upregulation of PBANKA_1443300 (MSP9) (3,4), as it suggests that red blood cell invasion by merozoites derived from liver, as opposed to blood, might have a mechanisms of red blood cell invasion independent of sialic acid.
- Surprisingly, genes were specifically upregulated in liver stage schizonts and detached cells included sbp1, mahrp1a and mahrp1b, which are indirectly involved in parasite sequestration during blood stages of infection (5).
- Among some genes predominantly expressed in developing pre-erythrocytic stages, an important finding was PBANKA_1003900, whose expression profile is as high as that of lisp1 and lisp2, although PBANKA_1003900 is currently annotated as being gametocyte-specific (6). The authors suggested re-naming it as lisp3.
Open questions and what I like about this preprint
It was for long thought that merozoites arising from liver and blood, were equal. It is only a very recent question to determine whether they are so. I like this work, because it opens a vast amount of questions that are barely beginning to be pondered in the malaria field. In their work, the authors showed that significant differences exist between liver-stage and blood-stage schizonts and merozoites. Altogether, that finding alone opens multiple questions including how the liver as a host organ, defines and modulates the outcome of infection in the blood stages, starting with the genetic makeup of the progeny that will give rise to the blood stages of infection. Specific to the work, open questions are:
- Can modulation of genes related to metabolism and fatty acid processing be used to alter organelle segregation and membrane formation, to reduce or even abolish merozoite formation in the liver?
- The authors found that an important difference exists between genes involved in egress in liver and blood schizonts. Assuming both types of egress mechanisms aim to ensure the maximal survival of merozoites, how could you alter merozoite survival by modifying egress mechanisms from the blood and liver?
- Since you found that MSP9 is not upregulated in liver schizonts, but is upregulated in blood schizonts, and since you suggest that this is related to sialic-acid dependent mechanisms of red blood cell invasion, why do you think this differs specifically between liver and blood-derived merozoites, and how is this relevant to the parasite’s life cycle?
- Converse to the above, you found that MAHRP1 and SBP1 were highly upregulated in liver schizonts/merozoites. You propose this is relevant in ensuring parasite sequestration and spleen remodelling immediately following liver egress, to ensure establishment of the parasite. However, the knock out parasites of both genes can survive in the blood albeit with lower replication rates, and despite being unable to sequester. Is there any other reason for MAHRP1 and SBP1 to be upregulated specifically in the liver-derived progeny?
- You found that a highly expressed gene in pre-erythrocytic stages is currently annotated as being gametocyte specific. Are there progeny merozoites arising from the liver, already committed to gametocytogenesis or capable of forming gametocytes immediately in the first RBC invasion without the need of a previous cycle? Could you manipulate commitment at the liver stages of infection to altogether abolish pathology? Conversely, are there individuals who have enhanced transmission potential immediately following liver egress?
- In your work, you used in vitro-derived parasites to analyse liver stages of infection. In vivo, different microenvironments can exist within the liver, including the different lobes, access to nutrients, oxygenation and vascularization. How do you think different microenvironments within the liver, influence a potential heterogeneity on the merozoite progeny? Furthermore, how does liver sensing of a whole body status (eg. pregnancy, immune status, nutrition state), influence the genetic makeup of the progeny merozoites produced during schizogony?
References
- Adapted from De Niz M, Burda PC, Kaiser G, del Portillo HA, Spielmann T*, Frischknecht F*, Heussler VT*, Progress in imaging tools: insights gained into Plasmodiumbiology, Nature Reviews Microbiology, (2017), 15(1):37-54
- Caldelari R, Dogga S, Schmid MW, Franke-Fayard B, Janse CJ, Soldati-Favre D, Heussler VT, Transcriptome analysis of Plasmodium bergheiduring exo-erythrocytic development, bioRxiv
- Li X, Chen H, Oo TH, Daly TM, Bergman LW, Liu S-C, et al. A Co-ligand Complex Anchors Plasmodium falciparum Merozoites to the Erythrocyte Invasion Receptor Band 3. J Biol Chem. (2004), 4;279(7):5765–71.
- Kariuki MM, Li X, Yamodo I, Chishti AH, Oh SS. Two Plasmodium falciparum merozoite proteins binding to erythrocyte band 3 form a direct complex. Biochem Biophys Res Commun.(2005), 4;338(4):1690–5.
- De Niz M, Ullrich AK*, Heiber A*, Blancke Soares A, Pick C, Lyck R, Keller D, Kaiser G, Prado M, Flemming S, del Portillo HA, Janse CJ, Heussler VT*, Spielmann T*, The machinery underlying malaria parasite virulence is conserved between rodent and human malaria parasites, Nature Communications, (2016), 7:11659.
- Deligianni E, Andreadaki M, Koutsouris K, Siden-Kiamos I. Sequence and functional divergence of gametocyte-specific parasitophorous vacuole membrane proteins in Plasmodium parasites. Mol Biochem Parasitol. Elsevier; (2018), 1;220:15–8.
doi: https://doi.org/10.1242/prelights.10446
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the bioinformatics category:
Enhancer-driven cell type comparison reveals similarities between the mammalian and bird pallium
Rodrigo Senovilla-Ganzo
Expressive modeling and fast simulation for dynamic compartments
Benjamin Dominik Maier
Transcriptional profiling of human brain cortex identifies novel lncRNA-mediated networks dysregulated in amyotrophic lateral sclerosis
Julio Molina Pineda
Also in the cell biology category:
Cell cycle-dependent mRNA localization in P-bodies
Mohammed JALLOH
Control of Inflammatory Response by Tissue Microenvironment
Roberto Amadio
Notch3 is a genetic modifier of NODAL signalling for patterning asymmetry during mouse heart looping
Bhaval Parmar
Also in the genetics category:
Enhancer-driven cell type comparison reveals similarities between the mammalian and bird pallium
Rodrigo Senovilla-Ganzo
Lipid-Based Transfection of Zebrafish Embryos: A Robust Protocol for Nucleic Acid Delivery
Roberto Rodríguez-Morales
Temporal constraints on enhancer usage shape the regulation of limb gene transcription
María Mariner-Faulí
Also in the microbiology category:
Characterization of natural product inhibitors of quorum sensing in Pseudomonas aeruginosa reveals competitive inhibition of RhlR by ortho-vanillin
UofA IMB565 et al.
Feedback loop regulation between viperin and viral hemorrhagic septicemia virus through competing protein degradation pathways
UofA IMB565 et al.
Lytic bacteriophages interact with respiratory epithelial cells and induce the secretion of antiviral and proinflammatory cytokines
UofA IMB565 et al.
preLists in the bioinformatics category:
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Also in the cell biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |
Also in the genetics category:
BSDB/GenSoc Spring Meeting 2024
A list of preprints highlighted at the British Society for Developmental Biology and Genetics Society joint Spring meeting 2024 at Warwick, UK.
| List by | Joyce Yu, Katherine Brown |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
Semmelweis Symposium 2022: 40th anniversary of international medical education at Semmelweis University
This preList contains preprints discussed during the 'Semmelweis Symposium 2022' (7-9 November), organised around the 40th anniversary of international medical education at Semmelweis University covering a wide range of topics.
| List by | Nándor Lipták |
20th “Genetics Workshops in Hungary”, Szeged (25th, September)
In this annual conference, Hungarian geneticists, biochemists and biotechnologists presented their works. Link: http://group.szbk.u-szeged.hu/minikonf/archive/prg2021.pdf
| List by | Nándor Lipták |
2nd Conference of the Visegrád Group Society for Developmental Biology
Preprints from the 2nd Conference of the Visegrád Group Society for Developmental Biology (2-5 September, 2021, Szeged, Hungary)
| List by | Nándor Lipták |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |
Also in the microbiology category:
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)