Large-scale curvature sensing by epithelial monolayers depends on active cell mechanics and nuclear mechanoadaptation
Posted on: 10 August 2020
Preprint posted on 4 July 2020
Article now published in Nature Physics at http://dx.doi.org/10.1038/s41567-021-01374-1
Sensing the curve: how epithelial cells modulate morphology and nuclear activity to adapt to curvature
Selected by Grace Lim, Ilaria Di MeglioCategories: biophysics, cell biology
Background
In native tissues, curvature is found everywhere. During tissue morphogenesis, cellular sheets deform via invaginations, budding and cavitation, amongst other processes, to generate 3D shapes like tubes and cysts of the lungs or kidneys, or the concave and convex curvature that characterize the crypts and the villi of the intestine. Curvature contributes structurally to most tissues, often by providing the increased surface area necessary for absorption like in the intestine, or gas exchange in the lungs. It is becoming increasingly clear that curvature also serves a functional purpose; curvature contributes to cell migration [1], to cell fate specification [2], and even to disease progression [3]. However, the underlying mechanisms by which cells sense local curvature and how it ultimately dictates cell behavior is less clear. In this study, Luciano et al. produce corrugated hydrogels with isotropic wavy patterns to investigate the effect of convex and concave curvatures on epithelial cells and nuclear shape.
Key findings
To understand how curvature of epithelial tissues can influence their functions, the authors first engineered corrugated hydrogels with well-defined curvature profiles. This was achieved using an optical photomask with alternating transparent and black stripes together with a photopolymerization method initiated by UV light (Fig. 1). Varying the width of the alternating stripes in the photomask enabled the generation of two types of corrugated substrates utilised in this study: P20 and P30, with sinusoidal patterns having 20mm and 30mm wavelengths respectively (Fig. 1). This approach enabled the authors to precisely control the wavelength and amplitude of the curved patterns, giving them a reliable system to answer their research questions.
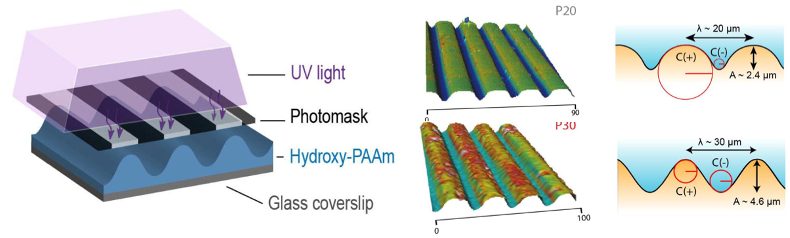
Figure 1. Generation of corrugated hydrogels. A photomask with alternating transparent and black stripes was used to produce patterns of UV light penetration to activate a photopolymerization reaction (left). Measurements of wavelength and amplitude from the P20 and P30 hydrogels (right). Adapted from Figure 1 of Luciano et al.
Epithelial cells grown on these corrugated substrates retained their overall epithelial morphology and cytoskeletal organization, maintaining their cell-cell interactions and characteristic hexagonal arrays. In contrast, cells exhibited differences in shape on flat vs. curved substrates. Cells on crests (concave) and valleys (convex) exhibited a mean 20% difference in thickness, wherein cells on crests extended along the tissue plane (squamous-like aspect ratio) while cells on valleys extended along the apico-basal direction (columnar-like aspect ratio). These findings suggest that curvature may be sensed by cells in an indirect manner by creating differences in thickness/density. To assess how curvature could create such cell shape modulations, Luciano et al., turn to a theoretical model.
The theoretical model used in this study is based on the vertex model, which has been used extensively to investigate the mechanisms and mechanics underlying epithelial tissue deformations [4]. Briefly, the vertex model describes a force balance equation that depends on three major contributions to the effective energy: a cell-substrate energy (proportional to the basal area), a cell-cell energy (proportional to the lateral area) and an energy coming from actomyosin-based tension (proportional to the apical perimeter). The authors adapt this model to their system by assuming that the basal area cannot detach from the substrate such as to remove the contribution of cell-substrate energy. Using numerical simulations to tune multiple parameters like wavelength, amplitude and tensions, and fitting these to experimental data, the analytical theory suggests that changes in substrate curvature into changes in cell thickness/density can be explained via a purely physical mechanism involving active contractile apico-basal tensions.
In addition to changes in cell shape, epithelial cells grown on corrugated substrates also display different nuclear morphologies, localization, and orientation. The nuclei of cells located in concave areas (crests) displayed an oblate shape, whereas those in convex regions (valleys) adopted a prolate shape – nuclear morphologies that can be attributed to changes in cell shape by an extension of the vertex model. Moreover, the authors found a bias in nuclear localization towards valleys where cell thickness is highest, and these nuclei also oriented more closely along the corrugation axis, as compared to nuclei of cells located in concave crest regions. Their theoretical model suggests that this could be an energy minimization step to reduce nuclear deformation resulting from cell shape changes and substrate curvature.
Based on the finding that substrate curvature modulates nuclear morphology, the authors next sought to investigate whether nuclear mechanics translates to changes in YAP localization. Recently, YAP localization was shown to depend on nuclear deformations. For instance, increased substrate stiffness physically deforms the nucleus and regulates the transport of YAP through nuclear pores [5], while circumferential actomyosin belt contraction induced by high cell density can drive nuclear export of YAP [6]. YAP may therefore sense curvature to induce the observed density changes. Indeed, YAP nuclear/cytoplasmic ratio was high on the side and top of P20 and P30 curved substrates, where cell density is low, whereas it was low on flat and bottom zones, where density is high. YAP therefore localizes preferentially to positively curved surfaces, where cell density is lower, suggesting that YAP-curvature sensing could occur indirectly from density-sensing. If this hypothesis is correct, then high cell densities – which increase the contributions of lateral tensions over apical tensions – should abrogate thickness modulations. Indeed, very dense epithelial tissues exhibit low YAP nuclear/cytoplasmic ratio, independently of substrate curvature. This confirmed that YAP curvature-sensing is inhibited at high cell density.
Given the well-known mechanosensing role of the nucleus [7], the authors went on to investigate a possible alteration in the nuclear lamina resulting from changes in nuclear morphology due to substrate curvature. Interestingly, the ratio of lamin A to lamin B differed depending on substrate curvature, with nuclei experiencing positive substrate curvature displaying lower lamin A levels and those experiencing negative substrate curvature with higher lamin B levels. The varying proportion of lamins A and B has been linked to elastic and viscous mechanical properties of the nucleus, which could be a mechanosensitive response of the cell to substrate curvature.
Finally, the authors sought to investigate whether curvature-induced modulation of nuclear shape, YAP and lamin A/B levels also affects DNA synthesis and proliferation. Nuclear volumes were lowest on the bottom of both P20 and P30 corrugated hydrogels, which suggests that negative/concave curvature, where density is highest, induces nuclear shape remodeling. By measuring chromatin condensation, which can be deduced from the nucleus (DAPI) intensity to nucleus volume ratio, the authors also found that chromatin condensation was associated with the nuclear deformation observed on the bottom of P20 and P30 zones. Chromatin compaction correlated with lower rates of proliferation observed in valleys, suggesting that negative curvatures induce chromatin condensation that in turn decreases DNA synthesis. Altogether, these results suggest that nuclear deformation observed on concave/negative curvature, which is associated with high cell densities, induce chromatin compaction and inhibit cell proliferation.
Why we like this preprint
It has remained difficult to study epithelial curvature without a robust system to generate well-defined curved substrate patterns for in vitro epithelial cell culture. Here, the authors engineer a way to precisely modulate substrate curvature to reliably address this question. This was combined with careful theoretical modelling, quantitative analysis of cell and nuclei morphologies, and the identification of key molecular players including YAP and lamins to establish an important link between curvature and mechanosensing pathways in epithelial tissues. We believe that this work presents important insights that can inform our understanding of many cellular and developmental contexts, given the pervasiveness of tissue curvature in a wide number of systems.
Questions to authors
- The authors suggest that curvature differences could be indirectly sensed via differences in cell density – cells in concave zones displayed greater cell areas, whereas convex zones were more densely packed. Is there a way to distinguish between changes in cell density induced by curvature versus that induced by other means, such as physical confinement of cells?
- The authors report curvature-induced changes in lamin levels and chromatin organization as separate observations. However, given that lamins and chromatin are known to closely interact, could the authors clarify whether changes in lamin levels can directly influence chromatin organization in their system?
- The authors propose that YAP curvature-sensing arises indirectly from cell density differences found between concave and convex curvatures. Have the authors investigated what may lie upstream of YAP regulation; is it nuclear deformation that physically promotes YAP nuclear exclusion, or are specific proteins (such as Merlin released from AJs) involved?
- The authors use “soft” corrugated hydrogels of stiffness 250kPa, and while this value is several orders of magnitude softer than glass/plastic culture dishes, it is higher than most soft tissues which have elastic moduli from 100Pa to 100kPa. The kidney, for instance, is >10kPa. Could a more physiological substrate stiffness affect the sensitivity of MDCK cells to thickness/density changes induced by curvature? Can the authors tune the stiffness of the corrugated hydrogels to investigate this ?
References
- Yevick, H.G., et al., Architecture and migration of an epithelium on a cylindrical wire. Proc Natl Acad Sci U S A, 2015. 112(19): p. 5944-9.
- Mobasseri, S.A., et al., Patterning of human epidermal stem cells on undulating elastomer substrates reflects differences in cell stiffness. Acta Biomater, 2019. 87: p. 256-264.
- Messal, H.A., et al., Tissue curvature and apicobasal mechanical tension imbalance instruct cancer morphogenesis. Nature, 2019. 566(7742): p. 126-130.
- Hannezo, E., J. Prost, and J.F. Joanny, Theory of epithelial sheet morphology in three dimensions. Proc Natl Acad Sci U S A, 2014. 111(1): p. 27-32.
- Elosegui-Artola, A., et al., Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell, 2017. 171(6): p. 1397-1410 e14.
- Furukawa, K.T., et al., The Epithelial Circumferential Actin Belt Regulates YAP/TAZ through Nucleocytoplasmic Shuttling of Merlin. Cell Rep, 2017. 20(6): p. 1435-1447.
- Kirby, T.J. and J. Lammerding, Emerging views of the nucleus as a cellular mechanosensor. Nat Cell Biol, 2018. 20(4): p. 373-381.
doi: https://doi.org/10.1242/prelights.23824
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the biophysics category:
Active flows drive clustering and sorting of membrane components with differential affinity to dynamic actin cytoskeleton
Teodora Piskova
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
Mitochondrial Fatty Acid Oxidation is Stimulated by Red Light Irradiation
Rickson Ribeiro, Marcus Oliveira
Also in the cell biology category:
Tissue mechanics and systemic signaling safeguard epithelial tissue against spindle misorientation
Ruoheng Li
Active flows drive clustering and sorting of membrane components with differential affinity to dynamic actin cytoskeleton
Teodora Piskova
A Novel Chimeric Antigen Receptor (CAR) - Strategy to Target EGFRVIII-Mutated Glioblastoma Cells via Macrophages
Dina Kabbara
preLists in the biophysics category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
66th Biophysical Society Annual Meeting, 2022
Preprints presented at the 66th BPS Annual Meeting, Feb 19 - 23, 2022 (The below list is not exhaustive and the preprints are listed in no particular order.)
| List by | Soni Mohapatra |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
Biophysical Society Meeting 2020
Some preprints presented at the Biophysical Society Meeting 2020 in San Diego, USA.
| List by | Tessa Sinnige |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Biomolecular NMR
Preprints related to the application and development of biomolecular NMR spectroscopy
| List by | Reid Alderson |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
Also in the cell biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
December in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cell cycle and division 2) cell migration and cytoskeleton 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Matthew Davies et al. |
November in preprints – the CellBio edition
This is the first community-driven preList! A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. Categories include: 1) cancer cell biology 2) cell cycle and division 3) cell migration and cytoskeleton 4) cell organelles and organisation 5) cell signalling and mechanosensing 6) genetics/gene expression
| List by | Felipe Del Valle Batalla et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |











 (No Ratings Yet)
(No Ratings Yet)