Polθ is phosphorylated by Polo-like kinase 1 (PLK1) to enable repair of DNA double strand breaks in mitosis
Posted on: 5 June 2023 , updated on: 8 September 2023
Preprint posted on 19 March 2023
Article now published in Nature at https://www.nature.com/articles/s41586-023-06506-6
The PLK1 kinase mandates DNA polymerase theta to safeguard the genome during mitosis.
Selected by Pierre CaronCategories: cell biology, molecular biology
Background
DNA double-strand breaks (DSBs) are highly cytotoxic lesions that if left unrepaired can lead to severe genomic instability or cell death. Our cells have evolved several repair mechanisms that deal with this type of damage, which are regulated across the cell cycle, by the chromatin landscape, and the configuration of broken ends (Scully et al., 2019).
One such pathway is Theta-Mediated End Joining (TMEJ) and its activity has been observed in several organisms including Arabidopsis thaliana, Caenorhabditis elegans, Drosophila melanogaster, Mus Musculus and Homo sapiens (Schimmel et al., 2019). This repair mechanism is by definition mutagenic as it relies on DNA polymerase theta (Polϴ, encoded by the gene POLQ) which uses the microhomology of the opposing 3′ single-stranded DNA to anneal and repair chromosomal breaks. This leads to specific genomic “scars”, such as deletions and templated insertions (Schimmel et al., 2017). Thus, the use of TMEJ strongly jeopardizes the integrity of our genome and may promote the onset of pathologies such as cancer.
A key question in the field is how cells can benefit from the use of such a repair mechanism? Interestingly, Polϴ activity protects from gross chromosomal rearrangements such as large DNA deletions (Roerink et al., 2014; van Schendel et al., 2016). In addition, POLQ deficiency increases sensitivity to ionizing radiation (Higgins et al., 2010) and micronuclei formation (Shima et al., 2004), implying that TMEJ supports the repair of DNA breaks that other pathways – such as non-homologous end joining (NHEJ) and homologous recombination (HR) – cannot support. In line with this hypothesis, it was shown that TMEJ is more active toward DSBs occurring in specific heterochromatic regions and blunt broken ends (Schep et al., 2021; Schimmel et al., 2017). On top of that, Polϴ deficiency confers synthetic lethality with a deficiency of genes encoding HR factors (Ceccaldi et al., 2015; Mateos-Gomez et al., 2015) and the Shieldin2 gene involved in NHEJ (Zatreanu et al., 2021b), arguing that TMEJ is a pathway operating in parallel with NHEJ and HR.
An alternative hypothesis would be that TMEJ remains operational when NHEJ and HR are not. Indeed, while the NHEJ and HR repair pathways are highly active during interphase, they are blocked during mitosis (Heijink et al., 2013; Orthwein et al., 2014). The active inhibition of NHEJ and HR during mitosis is orchestrated by the kinases Polo-like kinase 1 (PLK1) and Cyclin dependent kinase 1 (CDK1) which target and inhibit essential factors of these repair pathways such as 53BP1 and CtIP (Benada et al., 2015; Wang et al., 2018).
Conversely, the activity of the TMEJ repair pathway during mitosis has so far been little studied. Interestingly, a recent study has shown that synthetic DSBs incubated with mitotic cell extracts pull-down Polϴ, suggesting a role for TMEJ during this phase of the cell cycle (Heijink et al., 2022).
In this preprint (Gelot Camille et al., 2023), the Ceccaldi lab unveils that the kinase PLK1 licenses TMEJ-mediated DSB repair during mitosis by phosphorylating Polϴ thereby ensuring genome stability.
Key findings
Polϴ is recruited to mitotic DNA breaks
With the aim of characterizing the dynamics of Polϴ in response to DNA damage and its modes of regulation, the authors studied the foci formation of fluorescently-tagged Polϴ (endogenous or exogenous) in response to various genotoxic stresses that induce DNA double-strand breaks.
The authors found that Polϴ forms foci during S phase of the cell cycle in response to ionizing radiation (IR). Remarkably, cells in G2 or in mitosis (Fig1A) that had been exposed to replication stress during S phase showed an increase in Polϴ foci, suggesting that Polϴ binds DNA breaks during S phase and persists on those that are unrepaired and transmitted into mitosis. In addition, mitotic cells that were exposed to ionizing radiation displayed Polϴ foci, indicating that binding of Polϴ to mitotic DSBs is not restricted to breaks induced during S phase.
Polϴ repairs mitotic DNA breaks
Strikingly, the recruitment of Polϴ to mitotic DSBs was shown to rely on ATM, MDC1 and TOPBP1; which are factors described to accumulate in mitosis in response to DNA damage while NHEJ and HR factors do not. Furthermore, the authors demonstrated that, together with ATM, Polϴ regulates the mitotic checkpoint activation and is crucial to resolve Cas9- and IR-induced DSBs in mitosis. Finally, immunofluorescence colocalization experiments strongly suggested that Polϴ is not involved in mitotic DNA synthesis (MiDas). Collectively, the authors reveal that Polϴ defines an active and major pathway of DSB repair during mitosis.
PLK1 drives TMEJ in mitosis
The authors further investigated the mechanism by which Polϴ is regulated during the repair of DSBs in mitosis. Using several orthogonal approaches, they revealed that Polo-Like Kinase 1 (PLK1) directly phosphorylates Polϴ on several serine residues. Remarkably, phosphorylation of these serine residues and the activity of PLK1 are required for both recruitment of Polϴ to the sites of DNA damage in mitosis (Fig1 B) and the repair of DNA breaks. Thus, the authors unveiled that PLK1 licenses TMEJ-mediated DSB repair in mitosis while it inhibits both NHEJ and HR.
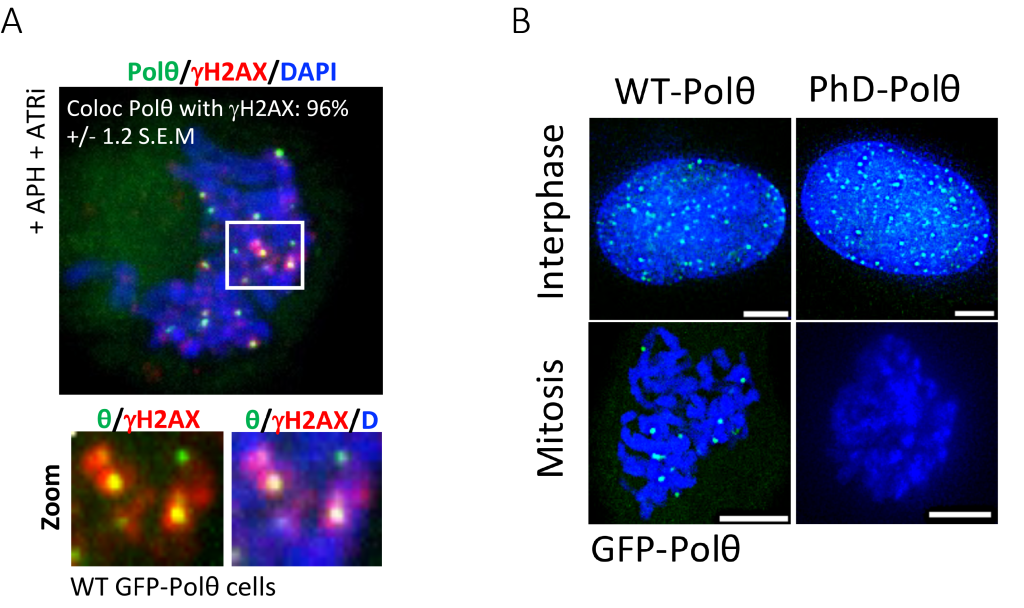
Figure 1. PLK1-dependent Polϴ phosphorylation drives its recruitment to mitotic DNA breaks (with panels from (Gelot Camille et al., 2023)). (A) Representative micrographs of immunofluorescence showing GFP-tagged Polϴ foci (green) localizing at mitotic DNA breaks (gammaH2A.X, Red) in human cells following replicative stress induced by the inhibition of ATR (ATRi). (B) Representative micrographs of either WT GFP-Polϴ or the GFP-PhD-Polϴ mutant in interphasic and mitotic cells. While the wild type of Poltheta (WT- Polϴ) is recruited to DNA breaks in interphase and mitosis, the mutant form that cannot be phosphorylated by PLK1 (PhD-Polϴ) is not recruited to mitotic DNA breaks.
TMEJ in mitosis safeguards genome stability
In addition, and in a remarkable way, the authors showed that PLK1-dependent TMEJ in mitosis prevents micronuclei formation, larger deletions, and that the synthetic lethality between POLQ and BRCA2 genes results from unrepaired DSB accumulation in mitosis. Thereby, the authors unveiled that Polϴ-mediated double-strand break repair protects against genomic instability during mitosis and that this pathway is required for the survival of cells deficient in HR.
Conclusion
By showing that Polϴ repairs DSBs during mitosis, the authors have redefined the physiological function of TMEJ. Often considered as an alternative and minor DNA repair pathway, the authors now reveal that DSB repair by Polϴ is the major pathway during mitosis. Despite its mutagenic nature, this pathway can be used by cells to take over mitotic or unrepaired DSBs from S/G2 phases to prevent chromosome rearrangements and to survive at the expense of small deletions.
References
Benada, J., Burdová, K., Lidak, T., von Morgen, P., & Macurek, L. (2015). Polo-like kinase 1 inhibits DNA damage response during mitosis. Cell Cycle, 14(2), 219–231. https://doi.org/10.4161/15384101.2014.977067
Ceccaldi, R., Liu, J. C., Amunugama, R., Hajdu, I., Primack, B., Petalcorin, M. I. R., O’Connor, K. W., Konstantinopoulos, P. A., Elledge, S. J., Boulton, S. J., Yusufzai, T., & D’Andrea, A. D. (2015). Homologous-recombination-deficient tumours are dependent on Polθ-mediated repair. Nature, 518(7538), 258–262. https://doi.org/10.1038/nature14184
Gelot Camille, Kovacs Marton Tibor, Miron Simona, Mylne Emilie, Ghouil Rania, Popova Tatiana, Dingli Florent, Loew Damarys, Guirouilh-Barbat Josée, Del Nery Elaine, Zinn-Justin Sophie, & Raphael Ceccaldi. (2023). Polθ is phosphorylated by Polo-like kinase 1 (PLK1) to enable repair of DNA double strand breaks in mitosis. BioRxiv. https://doi.org/https://doi.org/10.1101/2023.03.17.533134
Heijink, A. M., Krajewska, M., & van Vugt, M. A. T. M. (2013). The DNA damage response during mitosis. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 750(1–2), 45–55. https://doi.org/10.1016/j.mrfmmm.2013.07.003
Heijink, A. M., Stok, C., Porubsky, D., Manolika, E. M., de Kanter, J. K., Kok, Y. P., Everts, M., de Boer, H. R., Audrey, A., Bakker, F. J., Wierenga, E., Tijsterman, M., Gurvey, V., Spierings, D. C. J., Knipscheer, P., van Boxtel, R., Chaudhuri, A. R., Lansdorp, P. M., van Vugt, M. A. T. M. (2022). Sister chromatid exchanges induced by perturbed replication can form independently of BRCA1, BRCA2 and RAD51. Nat Commun 13, 6722 (2022). https://doi.org/10.1038/s41467-022-34519-8
Higgins, G. S., Prevo, R., Lee, Y.-F., Helleday, T., Muschel, R. J., Taylor, S., Yoshimura, M., Hickson, I. D., Bernhard, E. J., & McKenna, W. G. (2010). A Small Interfering RNA Screen of Genes Involved in DNA Repair Identifies Tumor-Specific Radiosensitization by POLQ Knockdown. Cancer Research, 70(7), 2984–2993. https://doi.org/10.1158/0008-5472.CAN-09-4040
Mateos-Gomez, P. A., Gong, F., Nair, N., Miller, K. M., Lazzerini-Denchi, E., & Sfeir, A. (2015). Mammalian polymerase θ promotes alternative NHEJ and suppresses recombination. Nature, 518(7538), 254–257. https://doi.org/10.1038/nature14157
Orthwein, A., Fradet-Turcotte, A., Noordermeer, S. M., Canny, M. D., Brun, C. M., Strecker, J., Escribano-Diaz, C., & Durocher, D. (2014). Mitosis Inhibits DNA Double-Strand Break Repair to Guard Against Telomere Fusions. Science, 344(6180), 189–193. https://doi.org/10.1126/science.1248024
Roerink, S. F., van Schendel, R., & Tijsterman, M. (2014). Polymerase theta-mediated end joining of replication-associated DNA breaks in C. elegans. Genome Research, 24(6), 954–962. https://doi.org/10.1101/gr.170431.113
Schep, R., Brinkman, E. K., Leemans, C., Vergara, X., van der Weide, R. H., Morris, B., van Schaik, T., Manzo, S. G., Peric-Hupkes, D., van den Berg, J., Beijersbergen, R. L., Medema, R. H., & van Steensel, B. (2021). Impact of chromatin context on Cas9-induced DNA double-strand break repair pathway balance. Molecular Cell, 81(10), 2216-2230.e10. https://doi.org/10.1016/j.molcel.2021.03.032
Schimmel, J., Kool, H., Schendel, R., & Tijsterman, M. (2017). Mutational signatures of non‐homologous and polymerase theta‐mediated end‐joining in embryonic stem cells. The EMBO Journal, 36(24), 3634–3649. https://doi.org/10.15252/embj.201796948
Schimmel, J., van Schendel, R., den Dunnen, J. T., & Tijsterman, M. (2019). Templated Insertions: A Smoking Gun for Polymerase Theta-Mediated End Joining. Trends in Genetics, 35(9), 632–644. https://doi.org/10.1016/j.tig.2019.06.001
Scully, R., Panday, A., Elango, R., & Willis, N. A. (2019). DNA double-strand break repair-pathway choice in somatic mammalian cells. Nature Reviews Molecular Cell Biology, 20(11), 698–714. https://doi.org/10.1038/s41580-019-0152-0
Shima, N., Munroe, R. J., & Schimenti, J. C. (2004). The Mouse Genomic Instability Mutation chaos1 Is an Allele of Polq That Exhibits Genetic Interaction with Atm. Molecular and Cellular Biology, 24(23), 10381–10389. https://doi.org/10.1128/MCB.24.23.10381-10389.2004
van Schendel, R., van Heteren, J., Welten, R., & Tijsterman, M. (2016). Genomic Scars Generated by Polymerase Theta Reveal the Versatile Mechanism of Alternative End-Joining. PLOS Genetics, 12(10), e1006368. https://doi.org/10.1371/journal.pgen.1006368
Wang, H., Qiu, Z., Liu, B., Wu, Y., Ren, J., Liu, Y., Zhao, Y., Wang, Y., Hao, S., Li, Z., Peng, B., & Xu, X. (2018). PLK1 targets CtIP to promote microhomology-mediated end joining. Nucleic Acids Research. https://doi.org/10.1093/nar/gky810
Zatreanu, D., Robinson, H. M. R., Alkhatib, O., Boursier, M., Finch, H., Geo, L., Grande, D., Grinkevich, V., Heald, R. A., Langdon, S., Majithiya, J., McWhirter, C., Martin, N. M. B., Moore, S., Neves, J., Rajendra, E., Ranzani, M., Schaedler, T., Stockley, M., … Lord, C. J. (2021a). Polθ inhibitors elicit BRCA-gene synthetic lethality and target PARP inhibitor resistance. Nature Communications, 12(1), 3636. https://doi.org/10.1038/s41467-021-23463-8
What I like about this preprint
Using multiple approaches, the authors defined a new mechanism for the regulation of DSB repair during mitosis by Polϴ. By showing that the survival of cells deficient in homologous recombination depends on this pathway, the authors open up new therapeutic perspectives for the use of PARP inhibitors in the treatment of tumors deficient for the BRCA1/2 genes, in particular by aiming at new targets in order to potentiate the efficacy of anticancer treatments.
Questions for the authors
Q1: In your study, you demonstrate that the conservation of PLK1-targeted Polϴ residues is required for the survival of BRCA2-deficient cells. This and other results thus demonstrate that synthetic lethality following BRCA2 and Polϴ depletion is due to a defect in DSB repair during mitosis. Naively, one might think that PLK1 deficiency combined with BRCA2 deficiency would also be lethal to cells. However, as mentioned in your study, PLK1 also inhibits NHEJ and HR in mitosis. So, do you know whether PLK1 inhibition or deficiency would mimic Polϴ deficiency in BRCA2-deficient cells?
Q2: In your study, you also show that Polϴ forms foci during S phase in wild-type cells (proficient for HR). In addition, Polϴ recruitment in S phase depends on several factors that regulate HR. Although HR and TMEJ are mutually exclusive repair pathways, it is tempting to think that these factors may ultimately activate TMEJ in the event that HR cannot proceed. Could you share your thoughts on these intriguing results? Do you think that factors involved in the SSA repair pathway could also stimulate the formation of Polϴ foci to DNA damage?
Acknowledgements
I thank Dr. Robin van Schendel (LUMC, Leiden, the Netherlands) and Dr. Reinier Prosée (Prelights team) for critical reading of the manuscript.
doi: https://doi.org/10.1242/prelights.34811
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Resilience to cardiac aging in Greenland shark Somniosus microcephalus
Theodora Stougiannou
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
Also in the molecular biology category:
A drought stress-induced MYB transcription factor regulates pavement cell shape in leaves of European aspen (Populus tremula)
Jeny Jose
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
preLists in the cell biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
December in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cell cycle and division 2) cell migration and cytoskeleton 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Matthew Davies et al. |
November in preprints – the CellBio edition
This is the first community-driven preList! A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. Categories include: 1) cancer cell biology 2) cell cycle and division 3) cell migration and cytoskeleton 4) cell organelles and organisation 5) cell signalling and mechanosensing 6) genetics/gene expression
| List by | Felipe Del Valle Batalla et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |
Also in the molecular biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |











 (3 votes)
(3 votes)