Rab11A regulates the constitutive secretory pathway during Toxoplasma gondii invasion of host cells and parasite replication
Posted on: 18 December 2019
Preprint posted on 25 September 2019
Article now published in PLOS Pathogens at http://dx.doi.org/10.1371/journal.ppat.1008106
Rab11A: a central player in the T. gondii constitutive secretory pathway, and in vital steps of the parasites’ journey into the target host cell.
Selected by Mariana De NizCategories: cell biology, microbiology
Background
Toxoplasma gondii is an obligate intracellular parasite of the genus Apicomplexa that is thought to affect 25-30% of the world’s human population (1). Beyond its clinical importance, T. gondii has an extraordinary capacity to infect any warm-blooded vertebrate host (2). Although T. gondii infections generally develop into a harmless latent form in most infected humans, in immunocompromised patients reactivation of a latent infection can be lethal. In addition to its relevance for public health, T. gondii has several features inherent to its biology, that have made it an important model organism to investigate intracellular host-pathogen interactions.
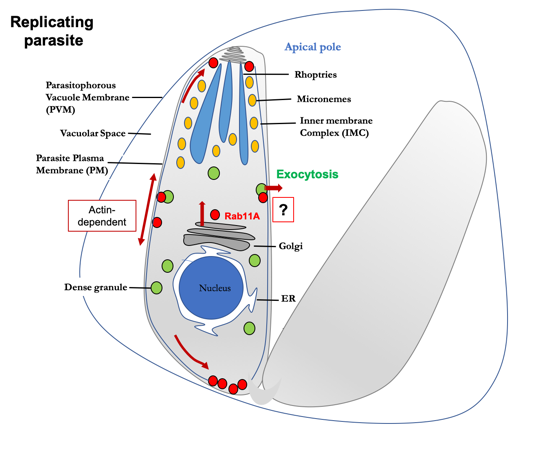
A research topic of great interest in the T. gondii field of work, is the investigation of secretory organelles, and the role they play in different stages of the parasite’s life cycle. Secretory organelles where virulence factors are stored include micronemes, rhoptries, and dense granules (Figure 1). Effective invasion of and egress from host cells relies on the temporally and spatially coordinated exocytosis of these secretory organelles. While the molecular mechanisms triggering rhoptry and microneme release have been well studied, (Reviewed in 3-5) dense granule secretion remains a poorly explored aspect of T. gondii vesicular trafficking. However, dense granules are known to play a crucial role in T. gondii development and survival.
In mammalian cells, the constitutive exocytic route supports sorting of newly synthesized proteins from the endoplasmic reticulum, through the Golgi, to the plasma membrane. In T. gondii, dense granules are considered to be the default constitutive secretory pathway. In their work, Venugopal et al (6) investigated the role of the small GTPase Rab11A in dense granule exocytosis, and its impact in key events in T. gondii entry to its target host cell, including adhesion, motility, and invasion (Figure 1).
Key findings and developments
General findings
- Rab11A regulates the constitutive secretory and recycling pathways, thus controlling secretion at the plasma membrane. Venugopal et al revealed an essential role of gondii Rab11A in:
- Promoting the cytoskeleton driven transport of dense granules, and the release of their content into the vacuolar space.
- Regulation of transmembrane protein trafficking and localization during parasite replication.
- Extracellular parasite motility and adhesion to host cells.
Novel tools generated in this study
- This study utilizes various tools to study Rab11A temporal and spatial distribution during the gondii life cycle, namely:
- A polyclonal antibody in mice against Rab11A (newly developed).
- A reporter Rab11A-mCherry parasite line which allows regulation of recombinant Rab11A levels using Shield-1.
- Two reporter lines co-expressing SAG1DGPI-GFP (the major gondii surface antigen truncated of its GPI anchor, which accumulates in dense granules), and mCherry-Rab11A (WT and DN) to explore dense granule dynamics in relation to Rab11A.
- A mathematical model of directed or diffusive motion to analyse vesicle dynamics.
- Transiently transfected Rab11A-WT and Rab11A-DN parasites with plasmids encoding the transmembrane HA-tagged glucose transporter 1 (GT1) or the Ty-tagged rhomboid protease 4 (ROM4).
Specific findings: Rab11A plays a role in dense granule exocytosis and transmembrane protein localization at the parasite plasma membrane
- Live microscopy in combination with various parasite markers for the actin cytoskeleton or the inner membrane complex led to several important conclusions (summarised in Figure 1) including:
- Rab11A participates in cargo transport between the apical and basal poles of the parasites and vesicular budding from the Golgi/ELC compartments.
- Rab11A-dependent vesicular transport depends on the actin cytoskeleton
- Rab11A may regulate actin-dependent material exchanges between parasites.
- Live imaging also showed that Rab11A-positive vesicles dynamically co-distribute with dense granules in replicating tachyzoites.
- Analysis of dense granule trajectories in Rab11A-WT and Rab11A-DN parasites suggested a role for Rab11A in regulating dense granule directed transport along the parasite cortical cytoskeleton. Rapid restoration of Rab11A functions by washing out Shield-1 and therefore the expression of Rab11A-DN suggested that Rab11A is also required for the steps of dense granule docking/tethering at the parasite plasma membrane.
- Accordingly, Rab11A-DN parasites display a drastic block in the release of several dense granule (GRA) proteins into the vacuolar space.
- The authors propose that Rab11A plays a broad role in exocytosis, and is involved in separate regulatory pathways: on one hand for the trafficking of proteins to the parasite plasma membrane; on the other hand, for the release of dense granule proteins into the vacuolar space during parasite replication.
Specific findings: Rab11A plays a role in host cell adhesion, motility and invasion
- A role for Rab11A in parasite entry into the host cell has been previously demonstrated. In the present study the authors investigated which steps of parasite entry (i.e. adhesion, motility, and invasion) were altered in Rab11A-DN parasites. They demonstrated that Rab11A-DN tachyzoites showed impaired attachment to human fibroblasts, as well as impaired motility correlated with altered morphology compared to their Rab11A-WT counterparts. However, despite the defects in adhesion and motility, Rab11A-DN parasites showed only mild defects in host cell invasion, and were able to successfully form a moving junction.
- Rab11A-DN parasites show impaired MIC2 secretion upon induction of microneme exocytosis by ethanol. The authors demonstrated that this was not the result of a defect in MIC2 protein synthesis or MIC2-positive microneme apical localisation.
- The authors went on to analyse Rab11A dynamic localization in extracellular motile parasites. They showed that Rab11A regulates the apically polarized accumulation of dense granules during the early steps of parasite adhesion and entry into host cells.
What I like about this paper
I found this an exciting piece, because it investigates a topic that seems relatively understudied in T. gondii, and the authors do this in a very elegant and step-wise manner. Equally, this study incorporates many modern tools to effectively answer relevant cell biological questions. I found the findings on Rab11A exciting and a promising topic for future work in this field. Furthermore, the research presented in this pre-print complements, and is coherent with, various recent and novel studies in T. gondii, successfully fitting a missing piece of a complex puzzle.
Open questions
*Note: questions with answers by authors are found at the bottom section of this highlight.
- You found in your work novel roles for Rab11A in T. gondii dense granule secretion, as well as parasite adhesion to the host cell, and motility. A very general question I have is that the previous study focusing specifically on Rab11A in T. gondii was published over a decade ago (7), with already promising findings and follow-up questions. In your work you are now addressing multiple roles of Rab11A. I am curious as to why the role of Rab11A in T. gondii seems to not have been a focus of research for a long time?
- Also a general question: you and others state that while the mechanisms of secretion for rhoptries and micronemes is well studied, constitutive secretion in T. gondii is a relatively understudied topic. Rab11A is only one member of this secretory pathway, and you have already shown exciting biology with relevance to pathology. Are you envisaging to address other members of the secretory pathway (eg. other Rabs or SNAREs), hypothesizing they might play equally important roles?
- You showed in your work that Rab11A dynamics suggest polarized transport of de novo synthesized material during daughter cell emergence and extracellular motility. Do you have a hypothesis on what the material being transported might be in each case, and whether it might be a different cargo specific to each of the processes?
- From the previous question, are you interested in investigating potential interactions between host and parasite organelles? Do you think this could shed further insights into the T. gondii constitutive secretory pathway and its effect and interactions with the target host cell?
- Given your findings, you suggest that Rab11A may contribute to the regulation of the actin network function and dynamics. Actin has been the focus of various labs, and has been shown to be key for multiple aspects of T. gondii biology. If Rab11A plays an important role in actin regulation, do you hypothesize this might expand the relevance of this Rab protein beyond the one you have studied/revealed in your work?
- You briefly mention a mode of cellular transport called hitchhiking, as a mechanism controlling organelle movement. Can you expand a bit further on this mechanism, specific to your findings and relevance in parasitology?
- Out of curiosity, do you also envisage studying membrane contact sites between T. gondii organelles and between T. gondii and the target host cell to further explore the role of Rab11A and the constitutive secretory pathway?
References
- Robert-Gangneux F., and Darde ML, Epidemiology of and diagnostic strategies for toxoplasmosis, Clin Microb Rev,2012, doi: 10.1128/CMR.05013-11.
- Dupont CD, Christian DA, and Hunter CA, Immune response and immunopathology during toxoplasmosis, Semin Immunopathol., 2012, doi: 10.1007/s00281-012-0339-3.
- Bullen HE, Bisio H, Soldati-Favre D, The triumvirate of signalling molecules controlling Toxoplasma microneme exocytosis: cyclic GMP, calcium, and phosphatidic acid, PloS Pathogens, 2019, doi: 10.1371/journal.ppat.1007670.
- Frenal K, Dubremetz JF, Lebrun M, Soldati-Favre D, Gliding motility powers invasion and egress in Apicomplexa, Nat Rev Microbiol, 2017, doi: 10.1038/nmicro.2017.86
- Dubremetz JF, Rhoptries are major players in Toxoplasma gondii invasion and host cell interaction, Cell Microb, 2007, doi: 10.1111/j.1462-5822.2007.00909.x
- Venugopal K., et al., Rab11a regulates the constitutive secretory pathway during Toxoplasma gondii invasion of host cells and parasite replication, bioRxiv, 2019, doi:10.1101/782391.
- Agop-Nersesian C, et al., Rab11a-controlled assembly of the inner membrane complex is required for completion of apicomplexan cytokinesis, Plos Pathogens, 2009, e1000270. doi:10.1371/journal.ppat.1000270
Acknowledgements
I thank Dr. Sabrina Marion and Dr. Kannan Venugopal for their engagement, time and input on this highlight, and for providing helpful insight and scientific discussions about their preprint. I thank also Mate Palfy for helpful comments on this highlight.
doi: https://doi.org/10.1242/prelights.15727
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Resilience to cardiac aging in Greenland shark Somniosus microcephalus
Theodora Stougiannou
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
Also in the microbiology category:
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Microbial Feast or Famine: dietary carbohydrate composition and gut microbiota metabolic function
Jasmine Talevi
Citrobacter rodentium infection activates colonic lamina propria group 2 innate lymphoid cells
André Luiz Amorim Costa, Marcus Oliveira
preLists in the cell biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
December in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cell cycle and division 2) cell migration and cytoskeleton 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Matthew Davies et al. |
November in preprints – the CellBio edition
This is the first community-driven preList! A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. Categories include: 1) cancer cell biology 2) cell cycle and division 3) cell migration and cytoskeleton 4) cell organelles and organisation 5) cell signalling and mechanosensing 6) genetics/gene expression
| List by | Felipe Del Valle Batalla et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |
Also in the microbiology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)