Vesicles driven by dynein and kinesin exhibit directional reversals without external regulators
Preprint posted on 28 September 2022 https://www.biorxiv.org/content/10.1101/2022.09.27.509758v1
Article now published in Nature Communications at http://dx.doi.org/10.1038/s41467-023-42605-8
To move or not to move: A novel in vitro assay successfully recreates intracellular bidirectional cargo transport, laying the foundations for further understanding
Selected by Divya PathakCategories: biochemistry, bioengineering, biophysics, cell biology
Background:
The cell is a very busy environment, wherein hundreds of molecules and vesicles are being transported at any given moment. Most intracellular cargoes show association with multiple types of motors raising the question– how is the directionality of cargo determined? In this preprint, D’Souza and colleagues try to answer this question.
Regulated intracellular transport is critical for proper cellular functioning, affecting processes like cell division, the positioning of organelles, and organelle transport. Intracellular transport can be long range using microtubules and short range using actin filaments. The microtubule-based motors Kinesin and Dynein carry cargo in opposite directions. What is surprising is that most intracellular cargo shows simultaneous association with multiple Kinesin and Dynein motors. Without a specific regulatory mechanism governing cargo transport, it is likely for the cargo to get stuck in limbo with both the motors engaged and undergoing futile ATPase cycles with no net movement. Alternatively, the cargo might randomly switch direction and end up in a place it’s not destined for. Intracellular cargoes like mitochondria, peroxisomes, endosomes, melanosomes and lipid droplets often move bidirectionally and regularly change direction en route to their final destinations.
Analyzing motor compositions on in vivo cargoes has been experimentally challenging not only due to difficulties in purifying a specific intracellular cargo type, but also the variation in motor-cargo associations over time. For most of the bidirectionally moving cargo, there are three models that can apply1. (1) Tug-of war, in which two oppositely directed motors simultaneously generate force and engage in a physical tug-of-war whose outcome decides the cargo’s direction2,3,4. In this model, changing the number of motors or their organization on cargo dictates the cargo’s net movement. (2) Exclusionary presence through motor association with and dissociation from the cargo. Adapter proteins on the cargo can interact with opposing motors and, depending on their interacting partner, the cargo either moves anterograde or retrograde5. This model prevents the futile use of ATP and ensures coordinated cargo movement without interference from the opposing motor. This model is also supported by the observation that motors exist in inactive states and that this autoinhibition is released upon cargo binding. (3) Activation by opposing motor, in which case the driving motor does not necessarily interfere, but rather activate the opposing motor by sheer stretching/force. It has been shown that inactivating one motor results in reduced motion by the opposing motor as well; disruption of either kinesin or dynein inhibits opposite-polarity vesicle transport6,7.
Until now, most of our insights into bidirectional transport have come from motors recruited to non-vesicular assemblies like DNA scaffolds, quantum dots, coverslips and beads4,8,13,14. These don’t recapitulate native cargo mostly due to the missing complexity of the lipid bilayer of the vesicular cargo9,10,11. Also, most of the reconstituted experiments couldn’t recapitulate the fast transport and directional reversals, and instead showed slowing down of motion and long stationary pauses. This suggested and provided increasing evidence for the exclusionary model in which dynein and kinesin are not active simultaneously on the same cargo. As most cargoes showed association with opposing motors, a model emerged in which external regulators or adaptors coordinate the activity of opposing motors to prevent their simultaneous activation.
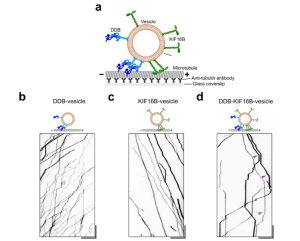
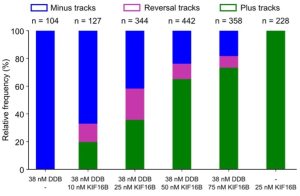
Key findings:
- In this study D’souza et al for the very first time successfully reconstituted bidirectional transport in vitro on liposomes that resemble the majority of the intracellular cargo (Fig. 1). They targeted purified motors – Dynein-Dynactin-BicD2 (DDB) complex and kinesin (KIF 16B) – to 132nm liposomes and could recapitulate the in vivo transport of cargoes including the fast unidirectional runs, intermittent pauses and directional reversals in the absence of any external regulatory protein.
- Cargo (liposome) pausing was enhanced when opposing motors were present. Not all pauses were the result of a tug-of-war as vesicles with only one kind of motor – either kinesin or dynein – exhibited processive runs with frequent pauses. The researchers titrated increasing amounts of kinesin (KIF16B) for the same amount of dynein (DDB) and could shift the balance in favor of kinesin-driven runs (Fig. 2). Also, in the pause phase, they observed elongation of cargo along the microtubule as seen for purified endosomes from Dictyostelium. These observations support the tug-of-war model.
- The successful reconstitution of bidirectional transport reported in this preprint also suggests that the presence of opposing motors doesn’t hinder the velocity of active driving motors nor slow down transport, reflected by the smooth unidirectional runs seen on DDB-KIF16B liposomes. Instead, presence of opposing motors results in longer pauses marked by the motors engaging in a tug-of war, followed by stochastic fluctuations that can lead to directional reversals without the need of external regulators.
- The researchers simulated different motor configurations to explain the bidirectional cargo transport observed experimentally. Their simulation predicts that 3 active motors drive a processive run and that the cargo pauses when 2 of those active motors detach along with the simultaneous attachment of one opposing motor. Pauses were characterized by a force balance between two active motors of opposing polarity and 4 inactive opposing motors (2 of each kind) to stabilize it. Transitioning from a pause to an active run in either direction is initiated by the stochastic detachment of the active motor of the opposing polarity or the attachment of two active motors. For cargo being driven by a low number of engaged motors, their simulation predicts that stochastic attachment or detachment of single motors determines the direction of cargo.
Why I chose this preprint?
Richard Feynman once said “What I cannot create, I do not understand”. Developing, or rather creating, this assay to recapitulate bidirectional motility marks the first attempt at improving our understanding of intracellular transport. This assay provides a foundation to build complexity in the form of adapter proteins, bilayer lipid composition etc. to dissect their role in intracellular transport. This is the first time that bidirectional motility has been reconstituted on liposomes which recapitulates the endogenous intracellular cargo. This assay marks the beginning of our ability to understand in vivo transport by trying to create it in vitro.
References:
- Hancock, W. Bidirectional cargo transport: moving beyond tug of war. Nat Rev Mol Cell Biol (2014); 15, 615–628
- Soppina V, Rai AK, Ramaiya AJ, Barak P, Mallik R. Tug-of-war between dissimilar teams of microtubule motors regulates transport and fission of endosomes. PNAS (2009); 106(46):19381-6
- Rezaul, K., Gupta, D., Semenova, I., Ikeda, K., Kraikivski, P., Yu, J., Cowan, A., Zaliapin, I. and Rodionov, V. Engineered Tug-of-War Between Kinesin and Dynein Controls Direction of Microtubule Based Transport In Vivo. Traffic (2016); 17: 475-486
- Derr ND, Goodman BS, Jungmann R, Leschziner AE, Shih WM, Reck-Peterson SL. Tug-of-war in motor protein ensembles revealed with a programmable DNA origami scaffold. (2012); 338(6107):662-5
- Fenton, A.R., Jongens, T.A. & Holzbaur, E.L.F. Mitochondrial adaptor TRAK2 activates and functionally links opposing kinesin and dynein motors. Nat Commun (2021); 12: 4578
- Steven P. Gross, Michael A. Welte, Steven M. Block, Eric F. Wieschaus; Coordination of opposite-polarity microtubule motors . J Cell Biol (2002); 156 (4): 715–724
- Shabeen Ally, Adam G. Larson, Kari Barlan, Sarah E. Rice, Vladimir I. Gelfand; Opposite-polarity motors activate one another to trigger cargo transport in live cells. J Cell Biol (2009); 187 (7): 1071–1082
- Toba S, Watanabe TM, Yamaguchi-Okimoto L, Toyoshima YY, Higuchi H. Overlapping hand-over-hand mechanism of single molecular motility of cytoplasmic dynein. PNAS (2006);103(15):5741-5
- Grover R, Fischer J, Schwarz FW, Walter WJ, Schwille P, Diez S. Transport efficiency of membrane-anchored kinesin-1 motors depends on motor density and diffusivity. PNAS (2016);113(46):E7185-E7193
- Li Q, Tseng KF, King SJ, Qiu W, Xu J. A fluid membrane enhances the velocity of cargo transport by small teams of kinesin-1. J Chem Phys. (2018);148(12):123318
- Nelson SR, Trybus KM, Warshaw DM. Motor coupling through lipid membranes enhances transport velocities for ensembles of myosin Va. PNAS (2014);111, 3986–3995
- Gina A. Monzon, Lara Scharrel, Ashwin D’Souza, Verena Henrichs, Ludger Santen, Stefan Diez; Stable tug-of-war between kinesin-1 and cytoplasmic dynein upon different ATP and roadblock concentrations. J Cell Sci (2020); 133 (22): jcs249938
- Belyy, V., Schlager, M., Foster, H. et al. The mammalian dynein–dynactin complex is a strong opponent to kinesin in a tug-of-war competition. Nat Cell Biol (2016);18, 1018–1024
- Allison M Gicking, Tzu-Chen Ma, Qingzhou Feng, Rui Jiang, Somayesadat Badieyan, Michael A Cianfrocco, William O Hancock Kinesin-1, -2, and -3 motors use family-specific mechanochemical strategies to effectively compete with dynein during bidirectional transport. eLife(2022); 11:e82228
Posted on: 25 January 2023
doi: https://doi.org/10.1242/prelights.33529
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the biochemistry category:
Structural basis of respiratory complexes adaptation to cold temperatures
Lens Placode Modulates Extracellular Matrix Formation During Early Eye Development
Generalized Biomolecular Modeling and Design with RoseTTAFold All-Atom
Also in the bioengineering category:
Scalable and efficient generation of mouse primordial germ cell-like cells
Multi-pass, single-molecule nanopore reading of long protein strands with single-amino acid sensitivity
Discovery and Validation of Context-Dependent Synthetic Mammalian Promoters
Also in the biophysics category:
Structural basis of respiratory complexes adaptation to cold temperatures
Actin polymerization drives lumen formation in a human epiblast model
Learning a conserved mechanism for early neuroectoderm morphogenesis
Also in the cell biology category:
Fetal brain response to maternal inflammation requires microglia
Alteration of long and short-term hematopoietic stem cell ratio causes myeloid-biased hematopoiesis
Clusters of lineage-specific genes are anchored by ZNF274 in repressive perinucleolar compartments
preLists in the biochemistry category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
Preprint Peer Review – Biochemistry Course at UFRJ, Brazil
Communication of scientific knowledge has changed dramatically in recent decades and the public perception of scientific discoveries depends on the peer review process of articles published in scientific journals. Preprints are key vehicles for the dissemination of scientific discoveries, but they are still not properly recognized by the scientific community since peer review is very limited. On the other hand, peer review is very heterogeneous and a fundamental aspect to improve it is to train young scientists on how to think critically and how to evaluate scientific knowledge in a professional way. Thus, this course aims to: i) train students on how to perform peer review of scientific manuscripts in a professional manner; ii) develop students' critical thinking; iii) contribute to the appreciation of preprints as important vehicles for the dissemination of scientific knowledge without restrictions; iv) contribute to the development of students' curricula, as their opinions will be published and indexed on the preLights platform. The evaluations will be based on qualitative analyses of the oral presentations of preprints in the field of biochemistry deposited in the bioRxiv server, of the critical reports written by the students, as well as of the participation of the students during the preprints discussions.
| List by | Marcus Oliveira |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
20th “Genetics Workshops in Hungary”, Szeged (25th, September)
In this annual conference, Hungarian geneticists, biochemists and biotechnologists presented their works. Link: http://group.szbk.u-szeged.hu/minikonf/archive/prg2021.pdf
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Also in the bioengineering category:
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Advances in microscopy
This preList highlights exciting unpublished preprint articles describing advances in microscopy with a focus on light-sheet microscopy.
| List by | Stephan Daetwyler |
Also in the biophysics category:
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
66th Biophysical Society Annual Meeting, 2022
Preprints presented at the 66th BPS Annual Meeting, Feb 19 - 23, 2022 (The below list is not exhaustive and the preprints are listed in no particular order.)
| List by | Soni Mohapatra |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
Biophysical Society Meeting 2020
Some preprints presented at the Biophysical Society Meeting 2020 in San Diego, USA.
| List by | Tessa Sinnige |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Biomolecular NMR
Preprints related to the application and development of biomolecular NMR spectroscopy
| List by | Reid Alderson |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
Also in the cell biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |











 (No Ratings Yet)
(No Ratings Yet)