An early cell shape transition drives evolutionary expansion of the human forebrain
Posted on: 31 July 2020
Preprint posted on 4 July 2020
Article now published in Cell at http://dx.doi.org/10.1016/j.cell.2021.02.050
How Humans Got Big Brains: A delayed cell-state transition in neurogenesis leads to a larger human brain
Selected by Monica Tambalo, Teresa Rayon, Maiko KitaokaCategories: developmental biology, evolutionary biology, neuroscience
Background
How has the human brain evolved its unique features? Such a fascinating question remains yet widely unexplored. Among the human brain’s remarkable features, its size is perhaps one of the most evident, with roughly a 3-fold increase in human brain size compared to chimpanzee and gorilla. A conspicuous amount of studies, mainly using rodent model systems, have contributed to our current understanding of brain embryonic development. Such approaches are excellent to teach us about conserved mechanisms but are not suitable for informing on human specific brain features and primate evolution.
A revolutionary approach to study human brain development and its evolution has been the advent of human brain organoids derived from human pluripotent stem cells [1],[2], and the invaluable possibility of extending this approach to virtually any species.
A fundamental process in brain development is neurogenesis. Within the cerebral cortex a neurogenic precursor, also known as neuroepithelial (NE) cell, is characterized by a columnar morphology and divides symmetrically during its proliferative phase. This proliferation is essential for the enlargement of the neocortex in primates. NE cells are highly epithelial, with tight and adherens junctions at their apical surface. NE progenitors will then transition into neurogenic radial glia (RG) by losing epithelial features, thinning and elongating the bipolar processes, and switching to asymmetric cell divisions, where one daughter cell self-maintains itself as RG and the other starts the neurogenic differentiation. This mechanism has been mainly studied using murine models while little is known about how it works in apes. Correlative evidence points to early changes in NE behaviour as a key mechanism for human cortical expansion, however direct evidence is poor.
To address directly this issue, Benito-Kwiecinski, Giandomenico, and colleagues [3] derived brain organoids from human, gorilla and chimpanzee and directly compared their neurogenetic properties, prior to neuron formation. This approach has given the authors the advantage of manipulating candidate genes and the ability to address their involvement in human and ape brain evolution.
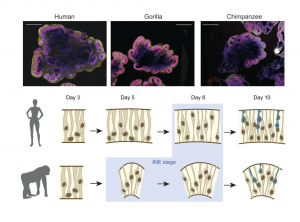 (Top panels) 5-week organoids stained for neural progenitor marker SOX2 (red), dorsal telencephalic/intermediate progenitor marker TBR2 (grey), neuronal markers TUJ1 (human) and HuCD (gorilla) in yellow, and DAPI (blue) showing human derived organoids become larger in overall size than gorilla and chimpanzee organoids. Scale bar: 1 mm. From Figure 1A.
(Top panels) 5-week organoids stained for neural progenitor marker SOX2 (red), dorsal telencephalic/intermediate progenitor marker TBR2 (grey), neuronal markers TUJ1 (human) and HuCD (gorilla) in yellow, and DAPI (blue) showing human derived organoids become larger in overall size than gorilla and chimpanzee organoids. Scale bar: 1 mm. From Figure 1A.
(Lower panels) Schematic summarizing the morphological changes in neural progenitor cells observed in human and gorilla organoids. Progenitor cells of both species undergo a gradual transition from NE to tNE to RG-like shapes. Human cells maintain columnar NE-characteristics for a longer period while gorilla cells show tNE morphologies (blue background) earlier than human. From Figure 2H.
Key points
- The authors developed comparable human, gorilla and chimpanzee brain organoids to study NE development prior to the onset of neurogenesis. Perhaps as expected, human brain organoids were consistently larger than the gorilla or chimp organoids.
- Human organoids show an enlarged ventricular apical surface at early stages of NE expansion, before the onset of neurogenesis.
- The transition from NE (non neurogenic) to RG (neurogenic) in human and ape occurs during several days, and ape NE cells make this transition more rapidly than human.
- Cell shape changes occur before the change in cell identity and before the onset of neurogenesis.
- Identification of a new intermediate cell morphology state, the tNE.
- By comparing RNA-seq data from each species, they focused on the differential expression of the ZEB2 gene, particularly due to its well-known role as a key mediator of the EMT transition and found that the ZEB2 gene drives the NE to RG transition.
- Delayed onset of ZEB2 expression extends the NE stage, compared to ape, and may be a major contributor to neocortical expansion in humans
- They generated an inducible-ZEB2 human brain organoid, demonstrating the powerful advantages of organoids to allow unprecedented control to manipulate genes involved in neurogenesis. By overexpressing human ZEB2 prematurely to match the expression levels and timing of gorilla organoids, the authors could trigger an earlier NE transition that led to a nonhuman, ape-like organoid morphology! This suggests that ZEB2 is a key regulator of species-specific brain development.
Things we like
This work elegantly demonstrates how the evolution of the neocortex can be studied systematically and quantitatively comparing stem cell models/organoids across species, providing perturbations that wouldn’t be feasible in human embryos. Previous 2D and 3D comparisons using stem cell models of primate cortical development had primarily focused on the differences in composition and proliferation rates of radial glial cells (RG) of macaque, human and chimpanzees [4]–[7]. Instead, this work centres its attention on the role of NE cells in cortical expansion for the first time and adds gorilla stem cells to the primate cerebral organoid zoo. Finally, we really appreciate that this work applies a classical embryology approach to organoids, where the authors start from a morphological observation and have begun to pin down the molecular mechanism.
Questions for the authors
Q1. How complex was it to establish reliable differentiation protocols for human, gorilla and chimpanzee brain organoids? And how consistent were the phenotypes observed across cell lines?
Q2. Is ZEB2 regulated differently in gorilla vs human vs chimp? How so? Ie, what triggers its expression, and how might this be regulated in a species-specific way?
Q3. Mutations in the ZEB2 gene cause Mowat-Wilson syndrome. Do the authors think that the early role that they uncovered for ZEB2 is related to the microcephaly and intellectual disability of the patients?
Q4. Mutations in the ZEB2 gene cause Mowat-Wilson syndrome. Do the authors think that the early role that they uncovered for ZEB2 is related to the microcephaly and intellectual disability of the patients?
Q5. Considering the exceptional current times, it has been challenging for everyone to work from home at a usual pace. How was the process of writing this preprint during lockdown?
References
- Lancaster, M. A.; Knoblich, J. A. Generation of Cerebral Organoids from Human Pluripotent Stem Cells. Nat. Protoc. 2014, 9 (10), 2329–2340. https://doi.org/10.1038/nprot.2014.158.
- Lancaster, M. A.; Renner, M.; Martin, C. A.; Wenzel, D.; Bicknell, L. S.; Hurles, M. E.; Homfray, T.; Penninger, J. M.; Jackson, A. P.; Knoblich, J. A. Cerebral Organoids Model Human Brain Development and Microcephaly. Nature 2013, 501 (7467), 373–379. https://doi.org/10.1038/nature12517.
- Benito-Kwiecinski, S.; Giandomenico, S. L.; Sutcliffe, M.; Riis, E. S.; Freire-Pritchett, P.; Kelava, I.; Wunderlich, S.; Martin, U.; Wray, G. A.; Lancaster, M. A. An Early Cell Shape Transition Drives Evolutionary Expansion of the Human Forebrain. bioRxiv 2020, 2020.07.04.188078. https://doi.org/10.1101/2020.07.04.188078.
- Neurogenesis, C.; Fiddes, I. T.; Lodewijk, G. A.; Mooring, M.; Salama, S. R.; Jacobs, F. M. J.; Haussler, D.; Fiddes, I. T.; Lodewijk, G. A.; Mooring, M.; Bosworth, C. M.; Ewing, A. D. Human-Specific NOTCH2NL Genes Affect Notch Article Human-Specific NOTCH2NL Genes Affect Notch Signaling and Cortical Neurogenesis. Cell 2018, 173 (6), 1356-1369.e22. https://doi.org/10.1016/j.cell.2018.03.051.
- Otani, T.; Marchetto, M. C.; Gage, F. H.; Simons, B. D.; Livesey, F. J.; Otani, T.; Marchetto, M. C.; Gage, F. H.; Simons, B. D.; Livesey, F. J. 2D and 3D Stem Cell Models of Primate Cortical Development Identify Species-Specific Differences in Progenitor Behavior Contributing to Brain Size Article 2D and 3D Stem Cell Models of Primate Cortical Development Identify Species-Specific Differences in Progenitor Behavior Contributing to Brain Size. Stem Cell 2016, 18 (4), 467–480. https://doi.org/10.1016/j.stem.2016.03.003.
- Suzuki, I. K.; Gacquer, D.; Heurck, R. Van; Polleux, F.; Detours, V.; Vanderhaeghen, P. Human-Specific NOTCH2NL Genes Expand Cortical Neurogenesis through Delta / Notch Regulation Article Human-Specific NOTCH2NL Genes Expand Cortical Neurogenesis through Delta / Notch Regulation. 2018, 1370–1384. https://doi.org/10.1016/j.cell.2018.03.067.
- Pollen, A. A.; Bhaduri, A.; Andrews, M. G.; Nowakowski, T. J.; Meyerson, O. S.; Mostajo-Radji, M. A.; Di Lullo, E.; Alvarado, B.; Bedolli, M.; Dougherty, M. L.; Fiddes, I. T.; Kronenberg, Z. N.; Shuga, J.; Leyrat, A. A.; West, J. A.; Bershteyn, M.; Lowe, C. B.; Pavlovic, B. J.; Salama, S. R.; Haussler, D.; Eichler, E. E.; Kriegstein, A. R. Establishing Cerebral Organoids as Models of Human-Specific Brain Evolution. Cell 2019, 176 (4), 743-756.e17. https://doi.org/10.1016/j.cell.2019.01.017.
doi: https://doi.org/10.1242/prelights.23633
Read preprintHave your say
Sign up to customise the site to your preferences and to receive alerts
Register hereAlso in the developmental biology category:
Tissue mechanics and systemic signaling safeguard epithelial tissue against spindle misorientation
Ruoheng Li
Human pluripotent stem cell-derived macrophages modify development of human kidney organoids
Theodora Stougiannou
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
Also in the evolutionary biology category:
Morphological variations in external genitalia do not explain the interspecific reproductive isolation in Nasonia species complex (Hymenoptera: Pteromalidae)
Stefan Friedrich Wirth
A high-coverage genome from a 200,000-year-old Denisovan
AND
A global map for introgressed structural variation and selection in humans
Siddharth Singh
Dissecting Gene Regulatory Networks Governing Human Cortical Cell Fate
Manuel Lessi
Also in the neuroscience category:
Imaging cellular activity simultaneously across all organs of a vertebrate reveals body-wide circuits
Muhammed Sinan Malik
Post-translational Tuning of Human Cortical Progenitor Neuronal Output
Jawdat Sandakly
Maturation of the glymphatic system confers innate resistance of the brain to Zika virus infection
Jimeng Li
preLists in the developmental biology category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
BSDB/GenSoc Spring Meeting 2024
A list of preprints highlighted at the British Society for Developmental Biology and Genetics Society joint Spring meeting 2024 at Warwick, UK.
| List by | Joyce Yu, Katherine Brown |
GfE/ DSDB meeting 2024
This preList highlights the preprints discussed at the 2024 joint German and Dutch developmental biology societies meeting that took place in March 2024 in Osnabrück, Germany.
| List by | Joyce Yu |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
2nd Conference of the Visegrád Group Society for Developmental Biology
Preprints from the 2nd Conference of the Visegrád Group Society for Developmental Biology (2-5 September, 2021, Szeged, Hungary)
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Society for Developmental Biology 79th Annual Meeting
Preprints at SDB 2020
| List by | Irepan Salvador-Martinez, Martin Estermann |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EDBC Alicante 2019
Preprints presented at the European Developmental Biology Congress (EDBC) in Alicante, October 23-26 2019.
| List by | Sergio Menchero et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |
Also in the evolutionary biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
EMBO | EMBL Symposium: The organism and its environment
This preList contains preprints discussed during the 'EMBO | EMBL Symposium: The organism and its environment', organised at EMBL Heidelberg, Germany (May 2023).
| List by | Girish Kale |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
COVID-19 / SARS-CoV-2 preprints
List of important preprints dealing with the ongoing coronavirus outbreak. See http://covidpreprints.com for additional resources and timeline, and https://connect.biorxiv.org/relate/content/181 for full list of bioRxiv and medRxiv preprints on this topic
| List by | Dey Lab, Zhang-He Goh |
1
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |
Also in the neuroscience category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |











 (No Ratings Yet)
(No Ratings Yet)
5 years
Sheila Garcia-Rosa
How long it took to develop this work, from the experimental design to the final experiment?