Three-axis classification of mouse lung mesenchymal cells reveals two populations of myofibroblasts
Posted on: 8 September 2021
Preprint posted on 4 August 2021
Article now published in Development at http://dx.doi.org/10.1242/dev.200081
Not just fillers. Narvaez del Pilar and Chen present a spatial classification of the mouse lung mesenchyme.
Selected by Sagar VarankarCategories: cell biology
Background:
Mesenchymal cells can be defined as the supportive niche in multiple tissues1–3. However, these cells continue to be poorly understood with respect to their molecular markers and functional capacities. A major obstacle for this has been their heterogeneity and is complicated by the presumed absence of a differentiation hierarchy. Considering their active role in homeostasis, regeneration, and disease progression, it is crucial to improve our understanding of the mesenchyme and its interaction with other cell populations.
The three-dimensional (3D) organization of organs generates specialized microenvironments within them where distinct cell populations can exist. The mammalian lung is an example of these tissue compartments, and can be broadly defined by the airways and alveoli4. While previous work has provided information about the lung epithelium, the respiratory field is yet to reach a joint consensus regarding the mesenchyme. Previous studies have identified subsets of mesenchymal cells and implicated them in regeneration and disease5–7. However, lineage markers identified for these cells are often shared between subsets which complicates their functional characterization. Some of the observed complexity can also be attributed to spatial cues that influence mesenchymal heterogeneity. An integration of molecular profiles with the positional information would lay a robust foundation for understanding the mesenchymal subsets.
In this preprint, the authors employ a multi-pronged approach to characterize the 3D heterogeneity of the pulmonary mesenchyme. Using single cell transcriptomics, lineage tracing and 3D immunostaining they define three distinct mesenchymal trajectories during development and growth of the mouse lung. They further make use of existing literature and imaging data to predict the functions of distinct mesenchymal subsets. Importantly, this study identifies a marker, Meox2, for the population of ‘interstitial fibroblasts’, which can serve as a signalling hub in the lung.
Key Findings:
Identification of a three-axes spatial heterogeneity of the lung mesenchyme:
| Cell Population | Markers |
| Pericytes | Pdgfrb |
| Vascular Smooth Muscle Cells (VSMC) | Acta2, Pdgfrb, Tagln |
| Airway Smooth Muscle Cells (ASMC) | Acta2, Actct1 |
| Myofibroblasts | Acta2, Pdgfra, Fgf18 |
| Co113a1 Matrix Fibroblasts | Wnt2, Col13a1 |
| Col14a1 Matrix Fibroblasts | Twist2, Col14a1 |
| Mesothelial Cells | Msln |
| Proliferating Cells | Mki67 |
| Table 1. Mesenchymal Cluster Markers |
| Table 1. Mesenchymal Cluster Markers |
Mesenchymal cells from mouse lungs were isolated via a negative gating strategy at distinct time points during development (E17, E19; E=embryonic) and growth (P7, P13, P20, P70; P=post-natal). Single cell RNA sequencing of these cells captured several pre-defined populations and identified their growth-associated dynamics. Based on preliminary analysis, specific markers were assigned to subset of the mesenchymal population (Table 1). The authors identified a proliferating cluster which was enriched in the developmental over post-natal stages. They also affirmed how Adrp, a well-noted lipofibroblast marker, exhibits heterogeneous expression trends and may not be the most specific gene for detecting this cell population. Using their single cell data and available literature on stromal cell-epithelium/-endothelium interactions the authors then developed a spatial annotation scheme for the mesenchyme. Subsets located closest to the distal lung epithelium were classified as the ‘epithelial mesenchyme’, cells in proximity of the endothelium were termed as the ‘vascular mesenchyme’ while stromal cells posited between these two compartments were annotated as the ‘interstitial mesenchyme’. Trajectory analysis confirmed the fate commitment of these cells over the course of development and the post-natal emergence of a supportive niche.
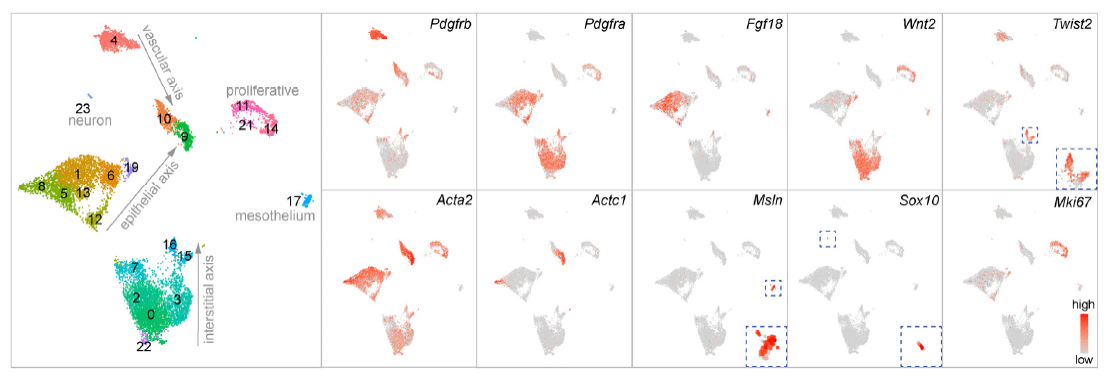
| Figure 1. Mesenchymal heterogeneity in the mouse lung. Single cell RNA sequencing data represented as UMAPs highlights the mesenchymal populations during development and growth of the mouse lung (Figure adapted from Figure 1b of Narvaez del Pilar and Chen). |
Stromal cell dynamics along the spatial axes:
Characterization of individual mesenchymal axes involved the re-clustering of the single cell transcriptomic data to predict growth associated dynamics of each subset, and its eventual validation with appropriate mouse models.
Vascular Axis: Additional analysis of this population identified four cell clusters which included vascular smooth muscle cells (VSMCs) and three subsets of pericytes viz., immature, proliferative and mature. The data also indicated that pericyte maturation, unlike VSMCs, was a post-natal event. Their spatial distribution and morphological differences were further validated with the PdgfrbCreER mouse line which labels the vascular mesenchyme.
Epithelial Axis: The three main stromal subsets identified in the epithelial axis comprised of the Pdgfra+ myofibroblasts, Cdh4/Hhip/Lgr6+ myofibroblasts and ASMCs. The authors identified two distinct subsets of Pdgfra+ myofibroblasts, which exhibited differences in overall expression (high or low) and subcellular localisation of this marker. Pdgfra-high cells associated with perinuclear localisation of the receptor and were situated around alveolar pockets, potentially involved in constriction during neonatal maturation. Interestingly, an onset of cell death pathways resulted in a loss of these cells in adult mice, thus highlighting their specific developmental role. On the other hand, Cdh4/Hhip/Lgr6+ myofibroblasts were situated proximal to the alveolar ducts and persisted in adult lungs despite their early developmental origin. These cells formed a continuum with the ASMCs and could wrap around alveolar ducts by virtue of their extended projections.
Interstitial Axis: In depth analysis identified Meox2 as a specific marker of the interstitial mesenchyme and localised them proximally within bronchovascular bundles. These cells also expressed low levels of non-perinuclear Pdgfra and could be labelled with the PdgfraCreER mouse model. Lineage labelling with the Myh11CreER mouse line, which labels alveolar myofibroblasts during embryonic development and neonatal growth did not label Meox2+ cells. These experiments affirmed that interstitial mesenchymal cells exist as an independent population and do not arise from the alveolar myofibroblasts.
Why I Chose This preprint?
Despite multiple studies focusing on it, there has been a lot of debate regarding the properties and functions of the lung mesenchyme. Several well-established markers often exhibit inconsistency across studies due to the limited understanding of how this cellular compartment changes in a spatial context. The multipronged approach employed in this study addresses this issue and provides a spatio-temporal map of distinct mesenchymal subsets. The functional capabilities predicted by the authors, in addition to their molecular signatures provide a roadmap for future studies involving the lung and its associated pathologies.
Questions For the Authors:
- How were the morphologies of distinct mesenchymal compartments defined? Will it be beneficial for future studies, to identify quantifiable features for them?
- Do you know if the abundance or distribution of these stromal compartments changes with age (more than 1 year old mice)?
- Do you intend to develop in vitro functional assays to further characterize these stromal cells and identify their potential to support different epithelial cell populations in co-cultures?
References:
- El Agha, E. et al. Mesenchymal Stem Cells in Fibrotic Disease. Cell Stem Cell 21, 166–177 (2017).
- Jiang, D. & Rinkevich, Y. Defining Skin Fibroblastic Cell Types Beyond CD90. Front. Cell Dev. Biol. 6, 1–3 (2018).
- McCarthy, N. et al. Distinct Mesenchymal Cell Populations Generate the Essential Intestinal BMP Signaling Gradient. Cell Stem Cell 26, 391-402.e5 (2020).
- Basil, M. C. & Morrisey, E. E. Lung regeneration: a tale of mice and men. Semin. Cell Dev. Biol. 100, 88–100 (2020).
- El Agha, E. et al. Two-Way Conversion between Lipogenic and Myogenic Fibroblastic Phenotypes Marks the Progression and Resolution of Lung Fibrosis. Cell Stem Cell 20, 261-273.e3 (2017).
- Biasin, V. et al. PDGFRα and αSMA mark two distinct mesenchymal cell populations involved in parenchymal and vascular remodeling in pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 318, L684–L697 (2020).
- Zepp, J. A. et al. Distinct Mesenchymal Lineages and Niches Promote Epithelial Self-Renewal and Myofibrogenesis in the Lung. Cell 170, 1134-1148.e10 (2017).
doi: https://doi.org/10.1242/prelights.30537
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Tissue mechanics and systemic signaling safeguard epithelial tissue against spindle misorientation
Ruoheng Li
Active flows drive clustering and sorting of membrane components with differential affinity to dynamic actin cytoskeleton
Teodora Piskova
A Novel Chimeric Antigen Receptor (CAR) - Strategy to Target EGFRVIII-Mutated Glioblastoma Cells via Macrophages
Dina Kabbara
preLists in the cell biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
December in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cell cycle and division 2) cell migration and cytoskeleton 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Matthew Davies et al. |
November in preprints – the CellBio edition
This is the first community-driven preList! A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. Categories include: 1) cancer cell biology 2) cell cycle and division 3) cell migration and cytoskeleton 4) cell organelles and organisation 5) cell signalling and mechanosensing 6) genetics/gene expression
| List by | Felipe Del Valle Batalla et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |











 (No Ratings Yet)
(No Ratings Yet)