Engineering functional human gastrointestinal organoid tissues using the three primary germ layers separately derived from pluripotent stem cells
Posted on: 14 August 2021
Preprint posted on 16 July 2021
Article now published in Cell Stem Cell at http://dx.doi.org/10.1016/j.stem.2021.10.010
Germ layer human gastrointestinal organoid tissues: A novel model system to understand stomach development.
Selected by Niveda UdaykumarCategories: developmental biology, molecular biology
Background
The stomach is a major organ of the gastrointestinal (GI) tract, and aids in the mechanical and chemical breakdown of ingested food. Like other organ development, the components of the stomach develop from the three germ layers. The endoderm forms the inner epithelial lining, the smooth muscle layer forms from the mesoderm, and the ectoderm gives rise to the enteric nervous system (ENS) – all three of which are necessary for the proper functioning of the stomach [1-4].
Signalling pathways play important roles in regulating morphogenesis, progenitor differentiation, and establishing regional identity during embryonic development [5]. Evidence from chick embryos indicates the presence of a reciprocal signalling module involving sonic hedgehog (Shh), which is secreted by epithelial cells and regulates BMP signalling in the adjacent mesenchyme [6-8]. This eventually results in proliferation/differentiation of the ENCCs (enteric neural crest cells) 9], and patterning, growth, and maturation of the stomach mesenchyme [10].
Although animal models facilitate the study of stomach development and disease, major structural and developmental differences exist in the stomach between different species [11]. Humans lack the forestomach found in rodents, and the avian gizzard is very different from the human stomach antrum [12]. In addition, mouse and chick animal models have accessibility and genetics difficulties, that limit the study of stomach development.
To overcome these issues, in this preprint, Eicher et. al., have generated a novel organoid system to recapitulate normal gastric development. This system consists of hPSCs (human pluripotent stem cells)-derived splanchnic mesenchyme and the ENCCs incorporated into human antral gastric organoids (hAGOs) and human fundic gastric organoids (hFGOs). This approach resulted in functional organoids with the epithelial glands innervated by smooth muscle. These functional organoids may be valuable in studying various aspects of gut development, including communication between germ layers, the role of human ENCCs during/in embryonic stomach development, and tissue morphogenesis.
Key findings
Generating three germ layer gastric organoids from hPSCs
The stomach tissue consists of glandular units of simple columnar epithelial cells surrounded by many layers of differently oriented smooth muscle layers. To best recapitulate the human stomach morphogenesis in vitro, the authors generated gastric organoids by incorporating enteric neural crest cells (ENCCs) and splanchnic mesenchyme (SM) with the hAGO spheroids [ENCCs+SM+hAGO], with each layer, derived separately from hPSCs. First,the neural crest cells (NCCs) were differentiated from RFP labelled human pluripotent stem cells (hPSCs) and incorporated with GFP labelled SM into hAGOs spheroids. Second, GFP-labelled splanchnic mesenchyme (SM) was generated from an hPSC line with constitutive GFP expression by a two-step process. The hPSCs were differentiated first into lateral plate mesoderm (LPM), as LPM can give rise to SM and cardiac fate. Treatment of LPM to retinoic acid (RA) induces SM fate, detected by the upregulation of the SM marker, FOXF1 followed by the downregulation of cardiac markers.
The spheroids were monitored for proper incorporation of the germ layers in the hAGOs in vitro followed by their transplantation into mice for 10-12 weeks for growth and maturation. Histological analysis of organoids showed well-organized smooth muscle layers with the distinct cellular architecture of the gastric tissue. In addition, the organoids also showed the characteristic glandular structures found in the human stomach.
The appearance of the gastric epithelial marker CLDN18 and the lack of intestinal epithelial marker CDH17, as identified by immunostaining, confirmed the gastric identity of the organoids. Additionally, the hAGO glands contained all expected cell types along with a neural network-like plexus embedded within the smooth muscle layer. Also, the 12-week hAGO was like the 38-week human gastric tissue, suggesting that the engineered three germ layer hAGO recapitulated the morphology and development of the human gastric tissue. Taken together, this suggests that the gastric organoids derived from the three germ layers separately may be a potential model system for the study of human stomach development.
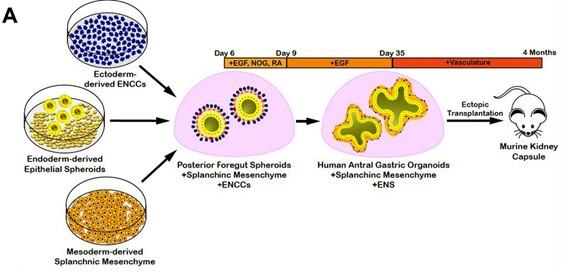
Figure 1: Schematic demonstrating the procedure to engineer three germ layer gastric organoids. (Adapted from Figure 2, Eicher et al., 2021).
The germ layer hAGOs display functional muscle contractions
The mechanical breakdown of food in the stomach occurs via contraction of the smooth muscles, which is controlled by the ENS. To investigate the ability of the organoid ENS and smooth muscle layer to form a neuromuscular junction, they monitored contractibility in an organ bath chamber. After a brief period of equilibration, the organoids showed spontaneous contractile oscillations. The oscillations demonstrated the presence of intramuscular interstitial cells of Cajal (ICCs), which was further supported by the expression of c-KIT, a marker of ICC, and the neuroglial cell marker TUJ1.
Next, the ENS of the engineered organoids were assessed for their ability to control gastric tissue contractions using electric field stimulation (EFS). EFS helps trigger neuronal firing followed by smooth muscle contractions. Administration of EFS pulses in the organoids resulted in an increase in their contractile activity. Treatment of the organoids with tetrodotoxin (TTX) resulted in a decrease in contractile activity, demonstrating that the ENS can regulate smooth muscle contractions in the engineered hAGO organoids.
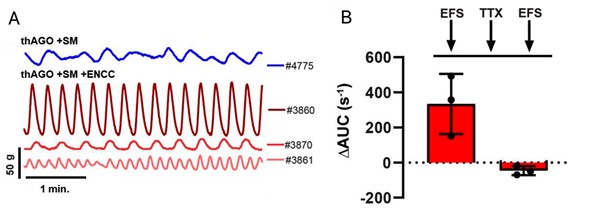
Figure 2: A. Contractile activity of isolated tissue from one thAGO+SM(blue) and three thAGO+SM+ ENCC (red) triggered by EFS. B. Loss of contractile activity triggered by EFS in the three germ layer organoids on treatment with TTX. (Adapted from Figure 4, Eicher et al., 2021).
The engineered hAGOs may be used to study the contribution of the three germ layers during gastric tissue development
To study this aspect of gastric tissue development, hPSCs were differentiated to migrating vagal-like ENCCs, recombined with hAGOs [ENCC+hAGO], and assessed for their ability to form the ENS in the absence of the exogenous mesenchyme layer. Although the ENCCs differentiated into different neuron subtypes with appropriate morphology, the spatial orientation and ENS development in these organoids were abnormal, suggesting that the mesenchyme is required for the proper spatial organization and ENS development during gastric tissue development. Transplantation of the ENCC+hAGO organoids in mice demonstrated that the ENCCs supported the growth and glandular morphogenesis of the organoid epithelium in vivo. Further, a time-course analysis of the transplanted organoids showed differentiated smooth muscle and neuroglial cells, demonstrating the contribution of the ENCCs during gastric tissue development. In addition, analysis of the organoid epithelium displayed similarities to Brunner’s glands, both morphologically and molecularly by combinatorial expression profiling. Brunner’s glands secrete sodium bicarbonate to neutralize gastric acids and are found in the submucosa. The absence of the mesenchyme and the added ENCCs induced a posterior fate to the gastric epithelium, possibly through BMP signalling. Culturing the organoids in the presence of noggin, a BMP signalling inhibitor, resulted in the absence of Brunner’s glands in the gastric epithelium. Taken together, the ENCCs are required for proper ENS development, growth, and production of posteriorizing factors such as BMP4 and BMP7.
Why I chose to highlight this preprint
I chose to highlight this preprint as I thought the authors used a clever approach to engineer hAGOs using the three germ layers and performed a comprehensive study to understand gastric tissue development. This preprint also beautifully unpicks the role of the individual germ layers in the development of gastric tissue. Additionally, generating functional organoids using this technique may open new avenues to engineer, study and understand the development of various tissues that are limited by the lack of appropriate animal model systems.
Questions to the authors
- How did the group come up with the idea of engineering the hAGOs with the three germ layers?
- What factor(s) in the mesenchyme do you think contributes to the precise spatial organization of the ENS during gastric tissue development?
References
1.Furness, J. B., Di Natale, M., Hunne, B., Oparija-Rogenmozere, L., Ward, S. M., Sasse, K. C.,
2. Powley, T. L., Stebbing, M. J., Jaffey, D. and Fothergill, L. J. (2020) ‘The identification of neuronal control pathways supplying effector tissues in the stomach’, Cell Tissue Res, 382(3) pp. 433-445.
3.Zhao, C. M., Martinez, V., Piqueras, L., Wang, L., Taché, Y. and Chen, D. (2008) ‘Control of gastric acid secretion in somatostatin receptor 2 deficient mice: shift from endocrine/paracrine to neurocrine pathways’, Endocrinology, 149(2), pp. 498-505.
4.Norlen, P., Ericsson, P., Kitano, M., Ekelund, M. and Hakanson, 1221 (2005) ‘The vagus regulates histamine mobilization from rat stomach ECL cells by controlling their sensitivity to gastrin’, J Physiol, 564(Pt 3), pp. 895-905.
5.Rydning, A., Lyng, O., Falkmer, S. and Grønbech, J. E. (2002) ‘Histamine is involved in gastric vasodilation during acid back diffusion via activation of sensory neurons’, Am J Physiol Gastrointest Liver Physiol, 283(3), pp. G603-11.
6.Le Guen, L., Marchal, S., Faure, S. and de Santa Barbara, P. (2015) ‘Mesenchymal-epithelial interactions during digestive tract development and epithelial stem cell regeneration, Cell Mol Life Sci, 72(20), pp. 3883-96.
7. Roberts, D. J., Johnson, R. L., Burke, A. C., Nelson, C. E., Morgan, B. A. and Tabin, C. (1995).’Sonic hedgehog is an endodermal signal inducing Bmp-4 and Hox genes during induction and regionalization of the chick hindgut’, Development, 121(10), pp. 3163-74.
8.Faure, S., de Santa Barbara, P., Roberts, D. J. and Whitman, M. (2002).Endogenous patterns of BMP signaling during early chick development, Dev Biol, 244(1), pp. 44-65.
9.De Santa Barbara, P., Williams, J., Goldstein, A. M., Doyle, A. M., Nielsen, C., Winfield, S., Faure, S. and Roberts, D. J. (2005) ‘Bone morphogenetic protein signaling pathway plays multiple roles during gastrointestinal tract development’, Dev Dyn, 234(2), pp. 312-22.
10. Nagy, N., Barad, C., Graham, H. K., Hotta, R., Cheng, L. S., Fejszak, N. and Goldstein, A. M. (2016) ‘Sonic hedgehog controls enteric nervous system development by patterning the extracellular matrix’, Development, 143(2), pp. 264-75.
11. Faure, S., McKey, J., Sagnol, S. and de Santa Barbara, P. (2015) ‘Enteric neural crest cells regulate vertebrate stomach patterning and differentiation, Development, 142(2), pp. 331-42.
12. de Santa Barbara, P., van den Brink, G. R. and Roberts, D. J. (2002) ‘Molecular etiology of gut malformations and diseases’, Am J Med Genet, 115(4), pp. 221-30.
13.Kim, T. H. and Shivdasani, R. A. (2016) ‘Stomach development, stem cells and disease’, Development, 143(4), pp. 554-65.
doi: https://doi.org/10.1242/prelights.30356
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the developmental biology category:
Tissue mechanics and systemic signaling safeguard epithelial tissue against spindle misorientation
Ruoheng Li
Human pluripotent stem cell-derived macrophages modify development of human kidney organoids
Theodora Stougiannou
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
Also in the molecular biology category:
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Loss of MGST1 during fibroblast differentiation enhances vulnerability to oxidative stress in human heart failure
Jeny Jose
Schistosoma haematobium DNA and Eggs in the Urine Sample of School-Age Children (SAC) in South-West Nigeria
Hala Taha
preLists in the developmental biology category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
BSDB/GenSoc Spring Meeting 2024
A list of preprints highlighted at the British Society for Developmental Biology and Genetics Society joint Spring meeting 2024 at Warwick, UK.
| List by | Joyce Yu, Katherine Brown |
GfE/ DSDB meeting 2024
This preList highlights the preprints discussed at the 2024 joint German and Dutch developmental biology societies meeting that took place in March 2024 in Osnabrück, Germany.
| List by | Joyce Yu |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
2nd Conference of the Visegrád Group Society for Developmental Biology
Preprints from the 2nd Conference of the Visegrád Group Society for Developmental Biology (2-5 September, 2021, Szeged, Hungary)
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Society for Developmental Biology 79th Annual Meeting
Preprints at SDB 2020
| List by | Irepan Salvador-Martinez, Martin Estermann |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EDBC Alicante 2019
Preprints presented at the European Developmental Biology Congress (EDBC) in Alicante, October 23-26 2019.
| List by | Sergio Menchero et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |
Also in the molecular biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |











 (No Ratings Yet)
(No Ratings Yet)