The vacuolar iron transporter mediates iron detoxification in Toxoplasma gondii
Posted on: 19 October 2021 , updated on: 16 August 2023
Preprint posted on 9 September 2021
Categories: cell biology
Updated 15 August 2023 with a postLight by Jennifer A Black
Congratulations to Aghabi and colleagues on their recent publication of the article “The vacuolar iron transporter mediates iron detoxification in Toxoplasma gondii” in Nature Communications (https://doi.org/10.1038/s41467-023-39436-y). In the peer-reviewed version, additional experiments have been performed to further support the author’s conclusions.
For example, in Figure1 the sub-cellular locations of both iron and zinc were compared to the locations of calcium and phosphorus. To do this, the authors used a technique called X-ray fluorescence microscopy (XFM) which combines X-rays with microscopy to image the elemental composition of a sample. Each element in the sample creates a specific fluorescent ‘footprint’ when exposed to an X-ray. Combined with microscopy, this allows researchers to locate where an element might be found within a cell. In Toxoplasma, the authors found limited overlap between iron and other elements leading to the conclusion that iron is stored separately. When they performed this experiment in the mutant cell lines that lacked the vacuolar iron transporter (Delta-VIT), they found generally less iron/more scattered signal in their mutants when compared to controls.
In Figure2, the authors show that loss of VIT leads to less plaques under increasing iron concentrations i.e., the parasites lacking VIT were more sensitive to iron and growing poorly compared to controls. Additionally, here they included a mutant in which they ‘added back’ (re-expressed) VIT in the cells lacking VIT (Delta-VIT) showing that the phenotypes reverted largely to that of control cells. This suggests that the effects they were seeing are more likely a direct result of VIT loss.
In Figure3, the authors show new images of the subcellular location of VIT using immunoelectron microscopy. They found that VIT appears to be located near a structure similar to a vacuole. In their preprint, they referred to this structure as the Vacuolar Associated Compartment (VAC), but in the final reviewed article, the VAC is instead referred to as the plant-like vacuolar compartment (PLVAC).
In Figure5, additional data to support the author’s conclusions have been added. They found, via flow cytometry, that their mutants (Delta-VIT) had elevated levels of reactive oxygen species in their mitochondria, but they didn’t observe a loss of mitochondrial potential. Instead, they found evidence that the parasites could upregulate a mitochondrial iron transporter (MIT) and perhaps use this strategy to limit the effect of higher iron concentrations in the cytosol, which could be toxic for the parasites.
Overall, the original conclusions presented in the preprint largely remain the same and have been strengthened by the inclusion of new data in the peer-reviewed, published paper. This study lays a strong foundation for further research into iron storage in these parasites, which is important to improve our understanding of how these parasites tackle stress arising from their environment.
Background
Iron is a very reactive metal, making it useful for biological reactions such as oxygen transport, but it also means iron reacts with other cellular metabolites leading to cell damage. For instance, iron reacting with hydrogen peroxide (H2O2) can form dangerous molecules called radicals which injure DNA and cellular structures like membranes. To avoid this, organisms store free iron. In mammals, iron is stored within the protein ferritin, whereas others (like yeast and plants) can pump free iron into membrane-bound structures (vacuoles) using transporters (such as the vacuolar iron transporter [VIT] in plants) (1). This difference in how iron is managed between mammalian cells and other organisms, including pathogens (disease-causing organisms), could be exploited to design better drugs targeting their ability to store iron. Toxoplasma gondii is a parasite which causes the disease Toxoplasmosis, a serious infection in immunocompromised individuals and for unborn babies. In the acute phase of disease, the parasites, known as tachyzoites, replicate and quickly lyse the host cells. In some cells, tachyzoites can differentiate into hard-to-treat cyst forms (bradyzoites), where they live for a long time and can transmit, for example, if the infected cell is eaten (i.e. undercooked or raw meat) (2).
Little is known about how T. gondii regulates and stores iron; a gap in the field the authors address within this study. By characterising the T. gondii homolog of the VIT (3), they show that VIT is involved in regulating iron storage in T. gondii, revealing how T. gondii respond to excess or scarce iron levels, and furthermore how iron management can contribute to the ability of T. gondii to cause disease in mammals.
Key Findings
In this study, the authors use CRISPR/Cas9 genome engineering to generate tachyzoite cells lacking VIT. This cell line forms the basis of their study.
A) T. gondii can use the VIT to store iron
VIT is non-essential for parasite survival in culture, but VIT-lacking cells do not grow as well as controls, i.e. the parasite replicates more slowly within host cells. The authors show that VIT loss renders parasites up to 25,000-fold more sensitive to excessive concentrations of iron.
To then ask if the VIT acts in storing iron, the authors depleted host cell iron using the iron-chelating compound deferoxamine (DFO), let the parasites invade then looked at how the parasites replicated. They show that the loss of the VIT compromises parasite replication. Furthermore, the parasites themselves contained less iron. Together, these data support a role for the VIT in iron storage and suggest that iron level regulation is important to support normal parasite growth.
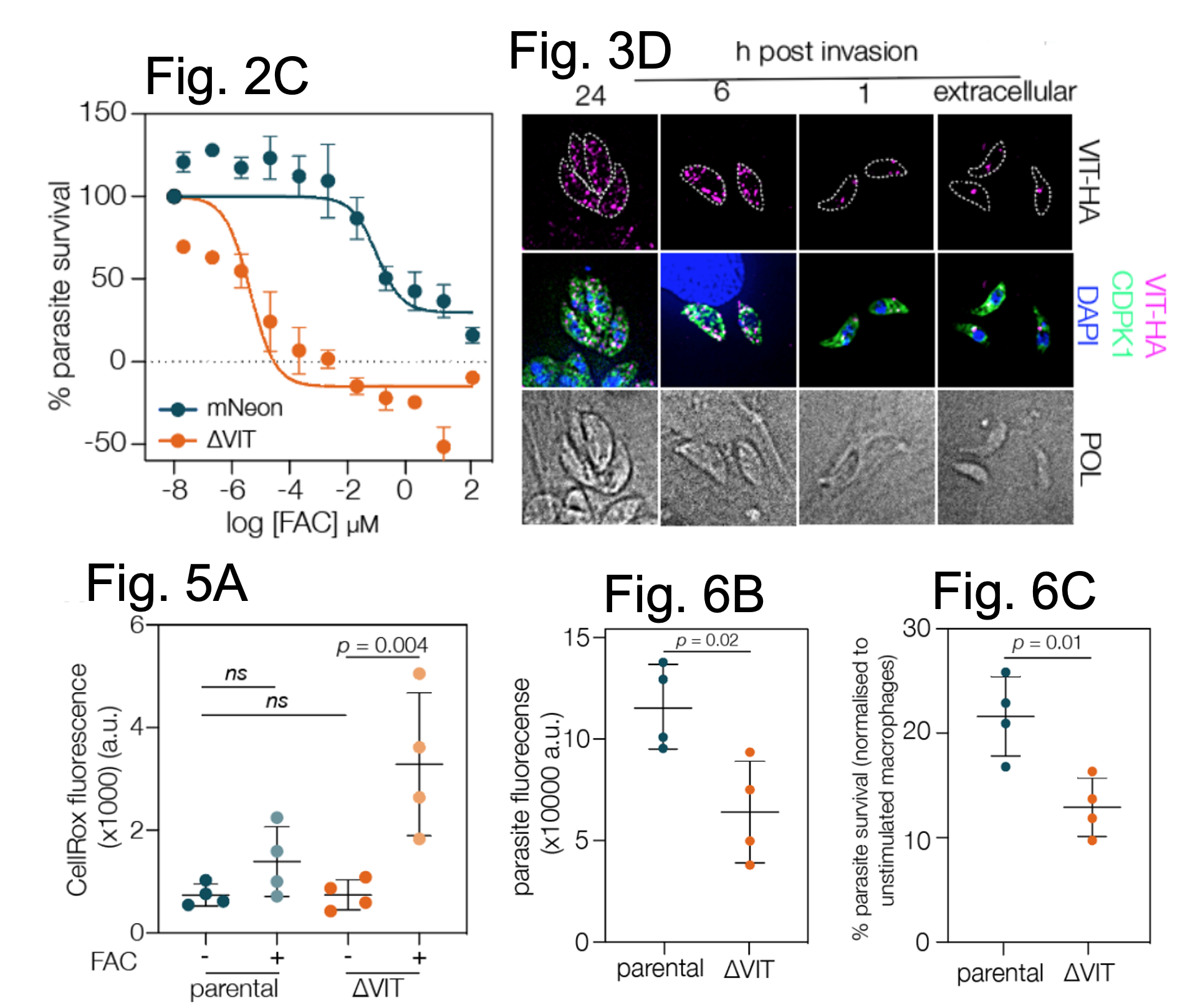
A selection of data from Aghabi et al. Fig, 2C shows that parasites lacking VIT are more sensitive to excessive levels of FAC relative to control cells. Fig. 3D shows the localisation of VIT across the lytic cycle of the parasite, starting extracellularly (singular foci) and ending at 24 hrs post host cell invasion ( fragmented). Scale bar = 5 μm. Fig. 5A shows that VIT cells have higher levels of CellROX Deep Red dye suggesting they have higher levels of ROS relative to controls. Fig. 6B and C show that though parasites lacking VIT can infect mouse macrophages, their survival is reduced relative to control cells. Figures adapted from Aghabi et al and reproduced under a CC-BY-NC-ND 4.0 International license.
B) VIT has a dynamic localisation
To locate the VIT in the parasites, the authors fused protein epitopes (an HA or myc tag) to the C-terminus of the endogenous protein then examined the location by high resolution microscopy. They found that in extracellular parasites (outside of a host cell), a single focus of signal appeared but once the parasites invaded host cells, several foci were seen. They also provide evidence that the VIT may localise to the Vacuolar Associated Compartment (VAC), which is an understudied organelle with similarities to a lysosome (i.e. a digestive organelle). The VAC contains proteases and now may also act as a putative site of iron storage in T. gondii.
C) External iron levels effects VIT expression
The authors then reasoned that the behaviour of the VIT may alter in response to changes in iron levels. They tested for possible changes by qRT-PCR (transcript levels), western blotting (protein levels) and the localisation by immunofluorescence (location). Under excess iron, both the VIT transcript and the protein levels become reduced, correlating with less VIT foci. Whereas, when iron was scarce, no changes in the transcript levels were seen, but paradoxically, less VIT protein was expressed, correlating with less VIT foci in the parasites. These data suggest that both excess and scarce iron levels alter the behaviour of the T. gondii VIT.
D) T. gondii can prioritise iron usage
Next, VIT-lacking cells and control cells, were subject to RNA-sequencing (RNAseq) to examine their transcriptomes. Interestingly, when looking at factors and pathways requiring iron, the authors found some differential expression of iron-associated pathways whereas for others, no significant changes were found in the absence of improper iron storage. Thus, some iron-associated pathways (and factors) can alter expression in response to changes in iron levels whereas others do not. As proposed by the authors, it is possible that T. gondii prioritises certain pathways over others when iron levels are dysregulated. In addition, up-regulation of a putative transporter involved in the removal of drugs from cells was observed, leading the authors to suggest T. gondii may remove excess iron from the cell if it is unable to store it correctly.
E) Dysregulated iron storage leads to oxidative stress
One reason as to why parasites lacking VIT grow poorly and are more sensitive to excess iron could be due to high levels of oxidative stress related to increased free iron reacting to produce reactive oxygen species (ROS). To test this, the authors exposed control and VIT lacking cells to excessive iron then measure the fluorescence of a dye (CellROX Deep Red) added to the cells. The signal of CellROX Deep Red corresponds to ROS in the cytosol. In support, they show that excess iron does induce more ROS in VIT-lacking parasites, but only in the cytosol and not under routine growth conditions. Thus, excessive, and incorrectly stored iron in T. gondii can cause more ROS to form which could reduce parasite survival, and though the authors found no changes at the transcript level for factors involved in resolving ROS damage, they did note that the activity of catalase (an enzyme which removes hydrogen peroxide) became enhanced in VIT lacking parasites under normal culture conditions.
In all, despite the parasites containing less iron, these data suggest the iron they do contain may be incorrectly stored and perhaps more freely available to react with other metabolites. As suggested by the authors, the increased catalase levels (and possibly the up-regulation of transporters) could help to explain why there is no significant increase in ROS levels (and a modest replication defect) in VIT-lacking cells in the absence of excess iron if the cells are able to remove iron-reactive metabolites or the iron itself and limit potential negative consequences.
F) VIT is needed for T. gondii virulence
Lastly, the authors asked if the VIT was required during a host infection. By infecting mice with control and VIT-lacking parasites, they were able to show that mice infected with VIT-lacking parasites survived better, presenting evidence that this loss of fitness in the host might be due to parasites failing to thrive in host macrophages.
What I Liked about this Preprint
On the one hand iron plays essential roles in most cells, whereas if left to its own devices, iron cause cell damage and even death. Understanding this fine balance between the good and the bad of iron is already an exciting field of research. What attracted me to this study was how surprisingly little we knew about iron related processes in this parasite and the curiosity to find out more. Something the authors also highlight as an interesting reason for studying iron storage in this parasite. Here, the authors have used interesting and informative experiments to ask key questions about iron storage in this parasite, now shedding light on this largely uncharacterised process in T. gondii. Though further work is needed to fully understand the intricacies of this process, their results will certainly create numerous new avenues of investigation into this process and open discussions about the roles of iron in this parasite. The more insight we gain into how pathogenic organisms operate, the higher the chance we can pinpoint key weaknesses which we can exploit for new therapies.
References
- Kaplan and D.M. Ward. The essential nature of iron usage and regulation. Current Biology (2013).
- Attias, D.E. Teixeira, M. Benchimol, R.C. Vommaro, P. Henrique Crepaldi and W. De Souza. The life-cycle of Toxoplasma gondii reviewed using animations. Parasites&Vectors (2020).
- Slavic, S. Krishna, A. Lahree, G. Bouver, K.K. Hanson, I. Vera, J.K. Pittman, H.M. Staines, M.M. Mota. A Vacuolar iron-transporter homologue acts as a detoxifier in Plasmodium. Nat. Comms (2016).
doi: https://doi.org/10.1242/prelights.30835
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
Kosmos: An AI Scientist for Autonomous Discovery
Roberto Amadio et al.
preLists in the cell biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
December in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cell cycle and division 2) cell migration and cytoskeleton 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Matthew Davies et al. |
November in preprints – the CellBio edition
This is the first community-driven preList! A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. Categories include: 1) cancer cell biology 2) cell cycle and division 3) cell migration and cytoskeleton 4) cell organelles and organisation 5) cell signalling and mechanosensing 6) genetics/gene expression
| List by | Felipe Del Valle Batalla et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |











 (No Ratings Yet)
(No Ratings Yet)