CRISPR-dependent base editing screens identify separation of function mutants of RADX with altered RAD51 regulatory activity
Posted on: 6 September 2023 , updated on: 19 January 2025
Preprint posted on 19 June 2023
Article now published in Journal of Molecular Biology at http://dx.doi.org/10.1016/j.jmb.2023.168236
Cracking the functional domains of the RADX gene during replication
Selected by Jessica Chevallier, Pierre CaronCategories: cell biology, genomics
Background
The recombinase RAD51 is an essential factor in maintaining genome stability by orchestrating the repair of DNA double-strand breaks (DSBs) by homologous recombination (HR) (Chakraborty et al., 2023; Kowalczykowski, 2015). Furthermore, RAD51 plays an essential role in maintaining the genome during replication and replicative stress. It promotes replication fork reversal at stalled replication forks and protects the fork ends from degradation (Bhat & Cortez, 2018). Moreover, RAD51 has been found to regulate replication fork restart, making it a central element in the cellular response to replicative stress (Mason et al., 2019).
As such, factors that regulate the binding of RAD51 to damaged replication forks or influence the activity of RAD51 are hypothesized to play a crucial role in RAD51-mediated genome integrity in response to replicative stress. For example, Breast Cancer Type 2 Susceptibility Protein (BRCA2) has been deemed an imported regulator that stimulates RAD51 nucleofilament formation at stalled replication forks, fork reversal and fork end protection (Chakraborty et al., 2023). In addition, Cortez and colleagues reported that the DNA single-strand binding protein RADX is a major player in the regulation of RAD51 at replication forks. Indeed, RADX depletion causes an aberrant increase in RAD51 activity, which reduces replication processivity and leads to aberrant DSB formation (Dungrawala et al., 2017). Conversely, RADX overexpression leads to nuclease-dependent fork degradation. It was discovered that RADX competes with RAD51 and limits the level of RAD51 at damaged replication forks. Ultimately, RADX regulates fork reversal and genome maintenance during replication and in response to replicative stress (Bhat et al., 2018).
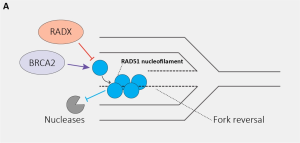
In this preprint, which follows up on a previous study showing that RADX binds to RAD51 and destabilized its nucleofilament form (Adolph et al., 2021), the Cortez Lab uses a CRISPR-dependent base editing approach to introduce mutations throughout the RADX gene and, thereby, characterize the role of RADX in genome stability (Madison B. Adolph et al., 2023). They identified motifs necessary for RADX function and uncovered that specific RADX mutants are still able to bind RAD51 but hinder its ATP hydrolysis activity.
Key findings
Cellular context dictates the impact of RADX inactivation
The authors were first of all interested in determining whether RADX inactivation results in a cell growth disadvantage. They implemented a two-color growth competition assay using an RPE-1 (Human Retinal Pigment Epithelial-1) immortalized cell line. RPE-1 wild-type (WT) cells either expressed GFP or mCherry transfected with siRNA targeting RADX gene (siRADX) or a non-targeting control siRNA (siNT). The results showed that while RADX depletion does not affect cell growth in the absence of P53, it is strongly affected in the presence of P53.
Previous studies demonstrated that RADX regulates the accumulation of RAD51 at replication forks, although the mechanisms behind how RADX promotes or inhibits RAD51 at these sites is highly dependent on the cellular context and remains unclear. Remarkably, depletion of RAD51 resulted in hypersensitivity to replication stress induced by hydroxyurea (HU). The inactivation of RADX, combined with RAD51 depletion (siRAD51) or its inhibition (B02), suppressed the hypersensitivity to replication stress induced by HU.
Deleterious RADX mutants identified using a CRISPR base editor screen
Madison Adolph and colleagues used a CRISPR base editor screen to introduce mutations via single-guide RNAs (sgRNAs) throughout the RADX gene and identified mutants conferring either a loss- or gain-of function. This experiment was performed using both RPE-1 cells proficient (WT) and deficient (p53-/-) for p53 as well as cells treated with HU and B02. The results were as follows: (1) sgRNAs were depleted in WT RPE-1 cells and those same sgRNAs were enriched in p53-/- RPE cells, (2) certain sgRNAs were enriched in both conditions at the RADX N-terminus and (3) sgRNAs targeting the amino acid residues 678-750 were depleted in both conditions.
The authors chose to focus on the mutations introduced in residues 678-750 because they were deleterious for cell growth. Using Alphafold, these mutations were predicted to be on the protein’s surface between the third and fourth oligosaccharide/oligonucleotide binding domains (OB), a previously uncharacterized RADX region (Fig 2A). The use of predictive tools to check if the mutations may be deleterious identified that variants S705N and E713K may be disruptive. Additionally, the variants E713K, D741N and K743Q have been implicated in TCGA (The Cancer Genome Atlas) studies.
The residues identified in the oligosaccharide/oligonucleotide binding domains (OB) are of physiological importance
To assess the physiological importance of the RADX residues 678-740, the authors determined the impact of their mutations – referred to as OB3-4 interdomain mutants or 3,4-ID mutants – on replication and in response to DNA damage. While these mutations did not affect the localization of RADX to replication forks, they considerably attenuated the elongation of replication forks and generated DNA damage during S phase. Furthermore, the expression of 3,4-ID RADX mutants in RADX deficient cells resulted in increased hypersensitivity to DNA damaging agents and replication stress.
The mutations identified lead to aberrant and toxic levels of RAD51 at the replication forks
Previous results from the Cortez lab and the analyses described above led the authors to investigate whether the phenotypes described earlier could result from a defect in the regulation of RAD51 during replication. While mutations did not alter the ability of RADX to bind single-stranded DNA, oligomerize and interact with RAD51, the authors observed that the effect of RADX mutations (3,4-ID) on the reduction of replication fork progression is mediated by RAD51. These results strongly suggest that the RADX mutants no longer regulate RAD51 levels at replication forks. Indeed, the authors observed aberrant levels of RAD51 at replication forks in cells expressing these mutants in an extent similar to those observed in RADX-deficient cells.
There is a defect in RAD51 ATPase activity in cells expressing 3,4-ID RADX mutants
In addition, and in a remarkable way, the authors found that the ATPase activity of RAD51 is impaired in cells expressing 3,4-ID RADX mutants (Fig 2B). Thereby, the authors revealed that these RADX mutants fail to stimulate RAD51 ATPse activity, which is crucial in the regulation of RAD51 at replication forks, for fork progression and in response to replicative stress.
Why this work is important?
Genomic instability is at the forefront of cancer initiation and progression. It has become paramount in cancer research to understand the mechanisms leading to defects in genome maintenance. RADX, the key protein in this study, modulates RAD51, a major player in DNA repair whose deregulation may pave the way towards tumorigenicity. Nevertheless, the mechanisms by which RADX promotes or inhibits RAD51 at fork sites remains unknown making this study by the Cortez Lab all the more important. The use of a CRISPR base editor screen to introduce mutations throughout the RADX gene is innovative and has allowed the researchers to pinpoint mutations in previously uncharted RADX regions. Further experiments using these mutants demonstrated that RADX stimulates RAD51 ATP hydrolysis, crucial for DNA replication and replicative stress responses. This surely adds another piece to the puzzle!
Questions for the authors
1. Do you expect the role of the OB3-4 interdomain to be conserved across species?
2. Do you envisage that the mutations you have identified in this study could be used as markers to predict the response of certain patients to the genotoxic agents used in cancer therapy?
3. RAD51 expression levels are altered in certain cancers. Do you think that expression levels of RADX (and by extension BRCA2) may also be a crucial factor in the regulation of RAD51 at replication forks?
References
(1) Adolph, M. B., Mohamed, T. M., Balakrishnan, S., Xue, C., Morati, F., Modesti, M., Greene, E. C., Chazin, W. J., & Cortez, D. (2021). RADX controls RAD51 filament dynamics to regulate replication fork stability. Molecular Cell, 81(5), 1074-1083.e5. https://doi.org/10.1016/j.molcel.2020.12.036
(2) Bhat, K. P., & Cortez, D. (2018). RPA and RAD51: fork reversal, fork protection, and genome stability. Nature Structural & Molecular Biology, 25(6), 446–453. https://doi.org/10.1038/s41594-018-0075-z
(3) Bhat, K. P., Krishnamoorthy, A., Dungrawala, H., Garcin, E. B., Modesti, M., & Cortez, D. (2018). RADX Modulates RAD51 Activity to Control Replication Fork Protection. Cell Reports, 24(3), 538–545. https://doi.org/10.1016/j.celrep.2018.06.061
(4) Chakraborty, S., Schirmeisen, K., & Lambert, S. A. (2023). The multifaceted functions of homologous recombination in dealing with replication-associated DNA damages. DNA Repair, 129, 103548. https://doi.org/10.1016/j.dnarep.2023.103548
(5) Dungrawala, H., Bhat, K. P., Le Meur, R., Chazin, W. J., Ding, X., Sharan, S. K., Wessel, S. R., Sathe, A. A., Zhao, R., & Cortez, D. (2017). RADX Promotes Genome Stability and Modulates Chemosensitivity by Regulating RAD51 at Replication Forks. Molecular Cell, 67(3), 374-386.e5. https://doi.org/10.1016/j.molcel.2017.06.023
(6) Kowalczykowski, S. C. (2015). An Overview of the Molecular Mechanisms of Recombinational DNA Repair. Cold Spring Harbor Perspectives in Biology, 7(11), a016410. https://doi.org/10.1101/cshperspect.a016410
(7) Madison B. Adolph, Atharv S. Garje, Swati Balakrishnan, Florian Morati, Mauro Modesti, Walter J. Chazin, & David Cortez. (2023). CRISPR-dependent base editing screens identify separation of function mutants of RADX with altered RAD51 regulatory activity. BioRxiv.
(8) Mason, J. M., Chan, Y.-L., Weichselbaum, R. W., & Bishop, D. K. (2019). Non-enzymatic roles of human RAD51 at stalled replication forks. Nature Communications, 10(1), 4410. https://doi.org/10.1038/s41467-019-12297-0
doi: https://doi.org/10.1242/prelights.35502
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Resilience to cardiac aging in Greenland shark Somniosus microcephalus
Theodora Stougiannou
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
Also in the genomics category:
Microbial Feast or Famine: dietary carbohydrate composition and gut microbiota metabolic function
Jasmine Talevi
A high-coverage genome from a 200,000-year-old Denisovan
AND
A global map for introgressed structural variation and selection in humans
Siddharth Singh
Human single-cell atlas analysis reveals heterogeneous endothelial signaling
Charis Qi
preLists in the cell biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
December in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cell cycle and division 2) cell migration and cytoskeleton 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Matthew Davies et al. |
November in preprints – the CellBio edition
This is the first community-driven preList! A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. Categories include: 1) cancer cell biology 2) cell cycle and division 3) cell migration and cytoskeleton 4) cell organelles and organisation 5) cell signalling and mechanosensing 6) genetics/gene expression
| List by | Felipe Del Valle Batalla et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |
Also in the genomics category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
Early 2025 preprints – the genetics & genomics edition
In this community-driven preList, a group of preLighters, with expertise in different areas of genetics and genomics have worked together to create this preprint reading list. Categories include: 1) bioinformatics 2) epigenetics 3) gene regulation 4) genomics 5) transcriptomics
| List by | Chee Kiang Ewe et al. |
End-of-year preprints – the genetics & genomics edition
In this community-driven preList, a group of preLighters, with expertise in different areas of genetics and genomics have worked together to create this preprint reading list. Categories include: 1) genomics 2) bioinformatics 3) gene regulation 4) epigenetics
| List by | Chee Kiang Ewe et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Semmelweis Symposium 2022: 40th anniversary of international medical education at Semmelweis University
This preList contains preprints discussed during the 'Semmelweis Symposium 2022' (7-9 November), organised around the 40th anniversary of international medical education at Semmelweis University covering a wide range of topics.
| List by | Nándor Lipták |
20th “Genetics Workshops in Hungary”, Szeged (25th, September)
In this annual conference, Hungarian geneticists, biochemists and biotechnologists presented their works. Link: http://group.szbk.u-szeged.hu/minikonf/archive/prg2021.pdf
| List by | Nándor Lipták |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |











 (1 votes)
(1 votes)