Mechanosensitive dynamics of lysosomes regulates the emergence of leader cells during collective cell migration
Posted on: 12 September 2022 , updated on: 15 November 2022
Preprint posted on 4 August 2022
What separates leaders from followers? Marwaha and colleagues reveal an unexpected role of lysosomes in leader cells during collective cell migration in their new study.
Selected by Jade ChanCategories: biophysics, cell biology
Background
Processes such as wound healing and organogenesis are critically dependent on the organised motion of cell clusters, termed collective cell migration1. During migration, a few cells at the outermost margin transform into leader cells with actin-rich lamellipodia1, 2. Leader cells are responsible for guiding followers to distant sites1, 2. The importance of leader cells in successful tissue development and wound healing is clear, but how do leaders emerge from a seemingly homogeneous mass of cells?
Previous studies have identified signaling proteins such as p53, Rho GTPases, and p120 catenin as markers of leader cells that distinguish them from followers3-6. Beyond these biochemical signatures, physical forces arising from cell-cell or cell-substrate interactions experienced by collectively moving cells appear to be influential in the acquisition of leader cell identity7, 8. However, how these diverse cues are integrated to promote leaders from followers remains to be solved.
Prior studies have emphasized the importance of dynamic rearrangements of the actin cytoskeleton during the adoption of leader cell identity7, 8. In contrast, relatively little attention has been paid to how cellular organelles are reorganized in leader cells. Aside from their established role in breaking down macromolecules, lysosomes have emerged as key players in cell motility. Studies suggest that spatial reorganization of lysosomes underlies migration of single fibroblasts and dendritic cells5, 9, but their role in collective migration is unknown.
Using a combination of live-cell imaging, optogenetics, and micropatterning, the authors uncovered a novel role of lysosomes in leader cells during collective cell migration. In epithelial monolayers and Drosophila embryos, they demonstrate that lysosomes amass at the cell periphery in cells located at the migrating front. This peripheral relocalization occurs before development of lamellipodia and blocking lysosomal relocalization disrupts leader cell emergence. Overall, the authors present a surprising role of lysosomes as integrators of biochemical and physical cues in leader cells.
Key Findings
Lysosomes accumulate peripherally in leader cells
To visualize lysosomes during collective cell migration, the authors grew MDCK or EpH4 epithelial cells to confluence inside migration chambers with removable inserts. These cells undergo collective migration after their confinement has been lifted. Immunostaining with lysosomal marker LAMP1 at different time points revealed peripheral accumulation of lysosomes in marginal cells. This accumulation was only observed in marginal cells, whereas lysosomes were present throughout the cytoplasm of follower cells. The peripheral occupancy of lysosomes (POL) was even greater in leader cells, which could be distinguished from marginal cells by the presence of lamellipodia.
To determine whether peripheral accumulation of lysosomes is conserved in vivo, the authors used a wound healing assay in D. melanogaster embryos. As observed in epithelial monolayers, lysosomes accrued peripherally in cells exhibiting lamellipodia at the migrating front, suggesting that the dynamic reorganization of lysosomes in emerging leaders is evolutionarily conserved.
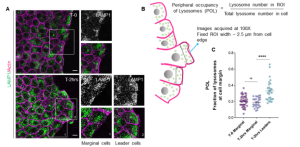
Figure 1. Immunostaining of lysosomal marker LAMP1 reveals accumulation at the periphery of marginal cells in collectively migrating epithelial cells.
Peripheral lysosome accumulation is required for leader cell identity
Next, they investigated whether lysosomal accumulation is necessary for leader cell emergence. To explore this, they used the Reversible Association of Motor Protein (RAMP) system10. In this system, the authors preferentially drove fluorescently tagged lysosomes toward the cell periphery or sequestered them near the nucleus. After being grown in a migration chamber, cells at the migration front were subjected to fluorescent time-lapse imaging. Strikingly, cells with peripheral lysosomes had a significantly greater proportion of leader cells compared to cells with perinuclear lysosomes. In fact, the proportion of leaders in cells with perinuclear lysosomes was even smaller than the baseline occurrence of leaders, and these cells exhibited peripheral actin cables and smaller focal adhesions, which are characteristic of follower cells.
Lysosomal accumulation is mechanosensitive and depends on actomyosin contractility
To determine how lysosomal reorganization occurs, the authors treated epithelial monolayers with blebbistatin or Y27632 to disrupt actomyosin contractility. After being allowed to migrate as sheets, the authors found that lysosomal relocalization was reduced and there was a lack of leader cells at the migrating front, suggesting that lysosome accumulation depends on force transmitted by the actin cytoskeleton.
To test the mechanosensitivity of lysosomal accumulation further, the authors adopted an optogenetic strategy to increase actomyosin contractility in specific cells within a monolayer11. Blue light stimulation resulted in either mitochondrial or plasma membrane localization of the RhoA activator ARHGEF11. When contractility was enhanced by localizing ARGHEF11 at the plasma membrane, they observed that lysosomes in adjacent cells gathered at the cell periphery away from the light-stimulated cell. Importantly, this finding suggests that physical forces transmitted via cell-cell interactions are key relocators of lysosomes. Furthermore, the authors cultured cells in micropatterned chambers with narrow beaks, which impart spatial cues to one or two cells trapped in the beak to become leaders. They observed lysosomal reorganization in cells trapped within beaks, indicating that concentrated mechanical force triggers peripheral lysosome accumulation.
Lastly, the authors demonstrated that peripheral lysosomes promote lamellipodium formation by sequestering inactive Rho GTPase Rac1 from the plasma membrane, enhancing actin remodeling.
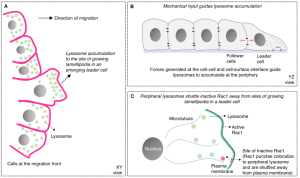
The figure above summarizes the proposed role of lysosomes in leader cell emergence during collective cell migration.
Why I chose this study
I was intrigued by this study because the idea that lysosomes are integrators of mechanical cues differs greatly from the canonical role of lysosomes as sites of catabolism. This study clearly demonstrates how much there is still to learn about organelles with ‘well-established’ functions. I was also interested in this study due to my research background in brain tumour mechanobiology and cell migration. It would be fascinating to see if lysosomal reorganization also underlies tumour cell invasion in different models of cancer, and whether metastatic potential is affected by the mechanical properties of different tissues. I also appreciated the multiple experimental paradigms the authors used to address their research questions.
Questions for the authors
- The models of collective cell migration used in this study mimic wound closure. Do you anticipate that lysosomal accumulation is conserved across different examples of collective cell migration such as gastrulation and tumour cell invasion?
- This study demonstrates that mechanical forces from cell-cell interactions promote peripheral lysosome accumulation. Is it possible for cell-substrate interactions to influence lysosome accumulation as well? Are lysosomal accumulation and leader cell emergence sensitive to substrate stiffness?
References
- Mayor R & Etienne-Manneville S (2016) The front and rear of collective cell migration. Nature Reviews Molecular Cell Biology 17(2):97-109.
- Brugués A, et al. (2014) Forces driving epithelial wound healing. Nature Physics 10(9):683-690.
- Reffay M, et al. (2014) Interplay of RhoA and mechanical forces in collective cell migration driven by leader cells. Nature Cell Biology 16(3):217-223.
- Peglion F, Llense F, & Etienne-Manneville S (2014) Adherens junction treadmilling during collective migration. Nature Cell Biology 16(7):639-651.
- Schiefermeier N, et al. (2014) The late endosomal p14-MP1 (LAMTOR2/3) complex regulates focal adhesion dynamics during cell migration. The Journal of Cell Biology 205(4):525-540.
- Kozyrska K, et al. (2022) p53 directs leader cell behavior, migration, and clearance during epithelial repair. Science 375(6581):eabl8876.
- Das T, et al. (2015) A molecular mechanotransduction pathway regulates collective migration of epithelial cells. Nature Cell Biology 17(3):276
- Vishwakarma M, et al. (2018) Mechanical interactions among followers determine the emergence of leaders in migrating epithelial cell collectives. Nature Communications 9.
- Bretou M, et al. (2017) Lysosome signaling controls the migration of dendritic cells. Science Immunology 2(16).
- Guardia CM, et al. (2019) Reversible association with motor proteins (RAMP): A streptavidin-based method to manipulate organelle positioning. PLoS Biology 17(5):e3000279
- Valon L, Marín-Llauradó A, Wyatt T, Charras G, & Trepat X (2017) Optogenetic control of cellular forces and mechanotransduction. Nature Communications 8:14396.
doi: https://doi.org/10.1242/prelights.32741
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the biophysics category:
Active flows drive clustering and sorting of membrane components with differential affinity to dynamic actin cytoskeleton
Teodora Piskova
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
Mitochondrial Fatty Acid Oxidation is Stimulated by Red Light Irradiation
Rickson Ribeiro, Marcus Oliveira
Also in the cell biology category:
Tissue mechanics and systemic signaling safeguard epithelial tissue against spindle misorientation
Ruoheng Li
Active flows drive clustering and sorting of membrane components with differential affinity to dynamic actin cytoskeleton
Teodora Piskova
A Novel Chimeric Antigen Receptor (CAR) - Strategy to Target EGFRVIII-Mutated Glioblastoma Cells via Macrophages
Dina Kabbara
preLists in the biophysics category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
66th Biophysical Society Annual Meeting, 2022
Preprints presented at the 66th BPS Annual Meeting, Feb 19 - 23, 2022 (The below list is not exhaustive and the preprints are listed in no particular order.)
| List by | Soni Mohapatra |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
Biophysical Society Meeting 2020
Some preprints presented at the Biophysical Society Meeting 2020 in San Diego, USA.
| List by | Tessa Sinnige |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Biomolecular NMR
Preprints related to the application and development of biomolecular NMR spectroscopy
| List by | Reid Alderson |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
Also in the cell biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
December in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cell cycle and division 2) cell migration and cytoskeleton 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Matthew Davies et al. |
November in preprints – the CellBio edition
This is the first community-driven preList! A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. Categories include: 1) cancer cell biology 2) cell cycle and division 3) cell migration and cytoskeleton 4) cell organelles and organisation 5) cell signalling and mechanosensing 6) genetics/gene expression
| List by | Felipe Del Valle Batalla et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |











 (No Ratings Yet)
(No Ratings Yet)