NAD(H) homeostasis is essential for host protection mediated by glycolytic myeloid cells in tuberculosis
Posted on: 16 October 2023
Preprint posted on 11 September 2022
Article now published in Nature Communications at https://www.nature.com/articles/s41467-023-40545-x#Sec32
Mycobacterium tuberculosis hijacks host myeloid cells metabolism by disrupting NAD+ homeostasis, impacting glycolysis, immune response and boosting infection.
Selected by Matheus Atella de Oliveira, Marcus OliveiraCategories: biochemistry, cell biology, microbiology
Updated 16 October 2023 with a postLight by Matheus Atella de Oliveira
This study was published in Nature Communications with a few updates and revisions. The authors have performed additional experiments investigating Mtb replication in LDHA knockout BMDMs. Although the absence of LDHA in BMDMs did not influence bacterial replication, when exposed to IFNγ, LDHA−/− macrophages displayed a minor rise in bacterial load.These findings emphasize the role of LDHA in an effective IFNy response.
The authors have also included supplementary data which introduces an isotype control for LDHA immunostaining, enhancing the reliability of their histological results.
Despite these additions and revisions, it’s worth noting that the interpretation of the results and the discussion of the findings have remained largely unchanged from those presented in the preprint version of the study.
Background
Tuberculosis (TB) is an infectious disease caused by the bacterial pathogen Mycobacterium tuberculosis (Mtb) that affects mainly the lungs and can be fatal, being one of the world’s deadliest infectious diseases1. This pathogen can cause an infection due to its capacity to modulate the immune response in its favor. However, the molecular mechanisms by which Mtb evades host phagocytes it’s still incomplete.
Myeloid cells are the main Mtb reservoir and represent a lineage of phagocytes with important roles in pathogens control. Over the past decade, there has been growing evidence suggesting that the function of myeloid cells in the immune response and their metabolism are intrinsically linked; For example, upon inflammatory stimuli, macrophages switch their metabolism to a glycolytic profile, with a disruption in the TCA cycle and a high lactate production2. It has already been shown that the downregulation of the glycolytic flux in myeloid cells impairs their function, interfering with the production of inflammatory mediators and phagocytosis3. Mtb is able to modulate the glycolytic flux in myeloid cells through an unclear mechanism, and the impact of this event on the immune response is unknown4.
During glycolysis, NAD+ is converted to NADH, which needs to be oxidized back to NAD+ for the regeneration of cellular energy. In immune cells, upon inflammatory signals, this process happens mainly through the activity of lactate dehydrogenase (LDH), culminating in lactate production. LDH is a tetramer composed of two subunits (LDHA and LDHB), where the presence of LDHA subunits favors the NADH re-oxidation while LDHB subunits favor the opposite reaction5. Despite its redox state, NAD+ levels can also be modulated during inflammatory diseases once the immune response promotes the expression of several NAD+ consuming enzymes, such as PARPs and CD38.
NAD+ synthesis pathways are dysregulated upon inflammatory signals, such as NAMPT, an enzyme that has a crucial role synthesizing NAD from its precursor nicotinamide (NAM)6. Therefore, the authors hypothesized that Mtb could affect myeloid cells NAD(H) homeostasis, impairing host cell glycolysis and allowing the infection. The study described in this preprint aimed to understand the mechanisms by which Mtb disrupts glycolysis and how it could impact the outcome of the infection.
Key findings
LDHA expression correlates with inflammatory and necrotic sites in human TB lung sections
Histoanalysis revealed consistent LDHA staining in early necrotic sites of lung tissues resected from TB patients, showing a reduced necrotic progression which was limited to the granulation layers. This suggests that the staining in the early lesions could be due to a release of LDHA by necrotic cells. The authors also detected LDHA expression in lymphoid aggregates, giant cells, macrophages, and lymphocytes. As these cells promote inflammation and no LDHA staining was observed in the vicinity of these cells, the authors raised the possibility that LDHA could be linked to immune function. Indeed, given that LDHA is the predominant LDH subunit expressed in myeloid cells, the staining of these cells in the inflammatory sites should be expected.
LDHA expression in myeloid cells is essential to protect mice against Mtb infection
The authors could show that the depletion of LDHA in myeloid cells increased the mortality of Mtb-infected mice and increased lung burden 10 weeks post-infection (wpi). While the lesions observed by histoanalysis in the lungs of wild-type (WT) mice were higher at early time points of the disease (4 wpi) and resolved by 30 wpi, mice lacking LDHA in myeloid cells (LDHALysM-/-) displayed worse lesions at 30 wpi.
LDHA genetic ablation in myeloid cells affects immune cell trafficking to the lungs in Mtb-infected mice
By using multiparameter flow cytometry, the authors analyzed the populations of immune cells present in the lungs of Mtb infected mice. Corresponding to the inflammatory profile observed in the histoanalysis, LDHALysM-/- mice exhibited a lower abundance of leukocytes at early time points (4 wpi). Interestingly the difference in the number of leukocytes was not restricted to the myeloid populations, but extended to lymphoid populations which were also decreased in LDHALysM-/- mice at 4 wpi. LDHALysM-/- mice also presented higher levels of the inflammatory cytokines MCP-1, RANTES, MIP-1a, and MIP-1b.
LDHA is required for macrophage’s metabolic response to IFN-γ
Although LDHALysM-/- mice were more susceptible to Mtb infection, transcriptome analysis found higher levels of IFN-γ signaling transcripts, which was not reflected in the protein levels. Therefore, the authors decided to investigate the capacity of LDHALysM-/- mice bone marrow-derived macrophages (LDHA BMDM) to respond metabolically to IFN-γ treatment. Based on ECAR analysis, the authors suggested that upon IFN-γ treatment WT BMDM glycolytic activity was induced to a greater extent in comparison to the LDHA-/- BMDM. Pyruvate addition rescued the deficit in the glycolytic response of LDHA-/- BMDM to IFN-γ probably by enhancing LDHB activity and promoting the oxidation of NADH, as LDH inhibition with GSK 2837808A attenuated the rescue. Although the NAD/NADH ratio was lower in the LDHA-/- BMDM compared to WT BMDM, glucose uptake was not affected. Metabolomic experiments revealed an accumulation of glycolysis and pentose phosphate pathway intermediates in the LDHA-/- BMDM, which was no longer present after pyruvate supplementation, suggesting that the glycolytic dysfunction was due to a lack of NAD+.
Mtb infection disrupts glycolysis by decreasing NAD(H) levels
The authors next hypothesized that the disruption of the glycolytic flux by the virulent Mtb infection – in comparison to inactive or attenuated Mtb – could be blunting macrophage’s response to IFN-y and inflammatory stimuli. Therefore, this event could increase macrophage’s susceptibility to Mtb infection. Although Mtb infection of macrophages enhanced the glycolytic flux in comparison to no stimuli, the glycolytic flux in the infected cells upon IFN-y treatment was lower than in uninfected cells. The authors also found a decrease in the accumulation of glycolysis and PPP intermediates and an increase in pyruvate and isocitrate levels upon Mtb infection. The authors suggest that the pyruvate accumulation and reduction in the glycolytic flux could be due to a lack of NADH to fuel LDH activity, as they also observed lower NAD(H) levels upon Mtb infection. Supplementation of BMDMs with the NAD precursor NAM partially restored NAD levels and rescued the glycolytic capacity of these cells.
Nicotinamide supplementation has therapeutic effects on tuberculosis by enhancing glycolysis
NAM supplementation has already been shown as an effective treatment for TB through an uncharacterized mechanism. The authors could show that NAM supplementation controls Mtb replication in BMDM macrophages in a dose-dependent manner. NAM treatment of bacterial cultures of Mtb reduced bacterial growth. NAM treatment of Mtb-infected macrophages was shown to reduce the bacterial burden, and this effect was reversed when combining the treatment with the glycolytic inhibitor 2-DG or with the NAMPT inhibitor FK866, showing that the therapeutical effects of NAM rely on its conversion to NAD and on the glycolytic activity. Experiments using the LDHA-/- BMDM supplemented with NAM should be considered to reinforce the suggestion that NAM supplementation and the glycolytic activity do not control bacterial burden through independent processes.
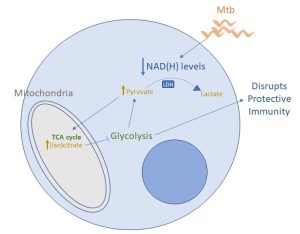
Figure 1: Mtb disrupts glycolysis in myeloid cells by depleting NAD(H) levels. Mtb infection leads to a depletion in NAD(H) levels, impairing LDH function and promoting the accumulation of pyruvate, which is metabolized by the Krebs cycle and promotes an increase in (iso)citrate levels, that can allosterically inhibit phosphofructokinase 1, disrupting the glycolytic flux and impairing immune response.
Why I think this preprint is important:
As bacteria are able to acquire resistance to antibiotics, new therapies must be studied and developed to treat bacterial infections. This preprint investigates the mechanisms by which Mycobacterium tuberculosis disrupts the glycolytic flux in myeloid cells, and the role of this process in the onset of the disease caused by this pathogen. It highlights the importance of NAD(H) homeostasis and suggests a potential therapeutic target for the treatment of the disease.
Questions and suggestions for the authors
1- Regarding the conclusion based on this first result, it would be more precise if the authors change the word “implicate” to “associate” as the authors did not show any direct role of LDH on the immune response and on the onset of human TB lesions. The authors could consider the possibility of assessing LDHB staining in TB lung sections to conclude that LDHA is the predominant isoform in areas of granulomatous inflammation.
2- LDHA genetic ablation in myeloid cells could affect cell viability as the depletion of this component of LDH has already been shown to induce apoptosis and decrease cell proliferation in cancer cells that also have a metabolism that relies on aerobic glycolysis7. Although we agree that the data regarding immune cells populations present in the lungs of LDHALysM-/- mice during Mtb infection indicates that cell viability was not affected, have you directly assessed cell viability and cell number of myeloid population in LDHALysM-/- mice? Alterations in myeloid cell number and viability could be the cause of the delay in the inflammatory process and cellular infiltration in LDHALysM-/- mice lungs. Therefore, perhaps you could consider a cell counting experiment and viability test of myeloid populations present in the blood and bone marrow of LDHALysM-/- mice.
3- Regarding the glycolytic capacity of LDHALysM-/- cells, the authors should carefully interpret the ECAR data as these might not directly reflect the glycolytic flux. ECAR could also be affected by CO2 produced by the TCA cycle8. A suggestion to the authors would be to use oxamate (a lactate dehydrogenase inhibitor) and fluorocitrate which disrupt the TCA cycle and to perform the ECAR experiments evaluating the contribution of glycolysis to the extracellular acidification. Lactate quantification experiments could also reinforce the glycolytic flux measurements.
4- Mtb infection in macrophages has already been shown to dysregulate the splicing machinery to promote non-productive RNAs or the translation of truncated proteins9. As you observed an increase in RNA levels of the IFN-y signaling pathway which did not translate to the proteins in Mtb-infected LDHALysM-/- mice, you might find it interesting to investigate RNA processing in future studies to better explain this unusual result.
5- the mouse sub-strain C57BL/6J is a mutant for nicotinamide nucleotide transhydrogenase, an enzyme that catalyzes the reduction of NADP at the expense of NADH oxidation in the mitochondria10,11. This mutation leads to abnormalities in the energy metabolism and in the redox balance of NAD derivatives10. It would be worth addressing how this mutation was considered in the overall conclusions of the study.
6- An alternative cytosolic pathway to regenerate NAD+ from NADH is the glycerol-3-phosphate (G3P) shuttle, which culminates in G3P production. You found an increase in G3P levels in LDHA-/- BMDM, however, you did not discuss the possible cause of it. Did you consider the possibility of the G3P shuttle compensating for the lack of LDHA in BMDM as you found an increase in G3P levels in this condition? Investigating this possibility could provide further insights into the metabolic changes.
7- Regarding the statistical analysis, it’s not clear if the samples follow a gaussian distribution. If so, to enhance the transparency of the statistical analysis, you could consider specifying which normality test was used in the materials and methods section. Alternatively, explaining the criteria for selecting specific statistical tests, even without assuming normal data distribution, would be beneficial.
References
- Bussi C, Gutierrez MG. Mycobacterium tuberculosis infection of host cells in space and time. FEMS Microbiol Rev 2019; 43: 341–361.
- Viola A, Munari F, Sánchez-Rodríguez R, Scolaro T, Castegna A. The Metabolic Signature of Macrophage Responses. Front Immunol 2019; 10: 1462.
- Soto-Heredero G, Gómez de las Heras MM, Gabandé-Rodríguez E, Oller J, Mittelbrunn M. Glycolysis – a key player in the inflammatory response. FEBS J 2020; 287: 3350–3369.
- Park J-H, Shim D, Kim KES, Lee W, Shin SJ. Understanding Metabolic Regulation Between Host and Pathogens: New Opportunities for the Development of Improved Therapeutic Strategies Against Mycobacterium tuberculosis Infection. Front Cell Infect Microbiol; 11, https://www.frontiersin.org/articles/10.3389/fcimb.2021.635335 (2021, accessed 20 June 2023).
- Osis G, Traylor AM, Black LM, Spangler D, George JF, Zarjou A, Verlander JW, Agarwal A. Expression of lactate dehydrogenase A and B isoforms in the mouse kidney. Am J Physiol Renal Physiol 2021; 320: F706–F718.
- Fang J, Chen W, Hou P, Liu Z, Zuo M, Liu S, Feng C, Han Y, Li P, Shi Y, Shao C. NAD+ metabolism-based immunoregulation and therapeutic potential. Cell Biosci 2023; 13: 81
- Zhang W, Wang C, Hu X, Lian Y, Ding C, Ming L. Inhibition of LDHA suppresses cell proliferation and increases mitochondrial apoptosis via the JNK signaling pathway in cervical cancer cells. Oncol Rep 2022; 47: 77.
- Mookerjee SA, Goncalves RLS, Gerencser AA, Nicholls DG, Brand MD. The contributions of respiration and glycolysis to extracellular acid production. Biochim Biophys Acta BBA – Bioenerg 2015; 1847: 171–181.
- Kalam H, Fontana MF, Kumar D. Alternate splicing of transcripts shape macrophage response to Mycobacterium tuberculosis infection. PLoS Pathog 2017; 13: e1006236.
- Ronchi JA, Figueira TR, Ravagnani FG, Oliveira HCF, Vercesi AE, Castilho RF. A spontaneous mutation in the nicotinamide nucleotide transhydrogenase gene of C57BL/6J mice results in mitochondrial redox abnormalities. Free Radic Biol Med 2013; 63: 446–456.
- Enríquez JA. Mind your mouse strain. Nat Metab 2019; 1: 5–7.
doi: https://doi.org/10.1242/prelights.35744
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the biochemistry category:
Active flows drive clustering and sorting of membrane components with differential affinity to dynamic actin cytoskeleton
Teodora Piskova
Snake venom metalloproteinases are predominantly responsible for the cytotoxic effects of certain African viper venoms
Daniel Osorno Valencia
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Also in the cell biology category:
Resilience to cardiac aging in Greenland shark Somniosus microcephalus
Theodora Stougiannou
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
Also in the microbiology category:
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Microbial Feast or Famine: dietary carbohydrate composition and gut microbiota metabolic function
Jasmine Talevi
Citrobacter rodentium infection activates colonic lamina propria group 2 innate lymphoid cells
André Luiz Amorim Costa, Marcus Oliveira
preLists in the biochemistry category:
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
Peer Review in Biomedical Sciences
Communication of scientific knowledge has changed dramatically in recent decades and the public perception of scientific discoveries depends on the peer review process of articles published in scientific journals. Preprints are key vehicles for the dissemination of scientific discoveries, but they are still not properly recognized by the scientific community since peer review is very limited. On the other hand, peer review is very heterogeneous and a fundamental aspect to improve it is to train young scientists on how to think critically and how to evaluate scientific knowledge in a professional way. Thus, this course aims to: i) train students on how to perform peer review of scientific manuscripts in a professional manner; ii) develop students' critical thinking; iii) contribute to the appreciation of preprints as important vehicles for the dissemination of scientific knowledge without restrictions; iv) contribute to the development of students' curricula, as their opinions will be published and indexed on the preLights platform. The evaluations will be based on qualitative analyses of the oral presentations of preprints in the field of biomedical sciences deposited in the bioRxiv server, of the critical reports written by the students, as well as of the participation of the students during the preprints discussions.
| List by | Marcus Oliveira et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
20th “Genetics Workshops in Hungary”, Szeged (25th, September)
In this annual conference, Hungarian geneticists, biochemists and biotechnologists presented their works. Link: http://group.szbk.u-szeged.hu/minikonf/archive/prg2021.pdf
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Also in the cell biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
December in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cell cycle and division 2) cell migration and cytoskeleton 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Matthew Davies et al. |
November in preprints – the CellBio edition
This is the first community-driven preList! A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. Categories include: 1) cancer cell biology 2) cell cycle and division 3) cell migration and cytoskeleton 4) cell organelles and organisation 5) cell signalling and mechanosensing 6) genetics/gene expression
| List by | Felipe Del Valle Batalla et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |
Also in the microbiology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)