Assembly of a persistent apical actin network by the formin Frl/Fmnl tunes epithelial cell deformability
Posted on: 24 July 2019 , updated on: 3 June 2020
Preprint posted on 23 June 2019
Article now published in Nature Cell Biology at https://www.nature.com/articles/s41556-020-0524-x
Formin’ the right shape: The formin Frl/Fmn1 modulates a persistent actin network that tunes epithelial cell deformations and enables the propagation of contractile forces to direct tissue-scale morphogenesis.
Selected by Melanie WhiteCategories: cell biology, developmental biology
Background
How small changes in cell shape are generated and transmitted across tissues to cause large-scale rearrangements of tissue architecture is a fundamental question in developmental biology. During various morphogenetic processes, actomyosin foci form at the apical cell cortex and undergo periodic contractions, or pulses. These actomyosin pulses pull on actin filaments connected to adherens junctions to drive changes in cell shape.
The mechanisms regulating actomyosin pulsation have been well studied and many of the underlying signalling molecules have been described1,2. Yet, it remained unclear how the myosin pulses interact with the cortical actin network to both effect changes in shape within the cell, and propagate forces across cell boundaries throughout a tissue.
In this preprint by Dehapiot et al, the authors identify two subpopulations of cortical actin filaments in Drosophila embryos: a pulsatile network and a persistent network. By combining genetic manipulations, live imaging and computational modelling, they show how the pulsatile network promotes cell shape changes and the persistent network supports the transmission of contractile forces through the tissue.
Key findings
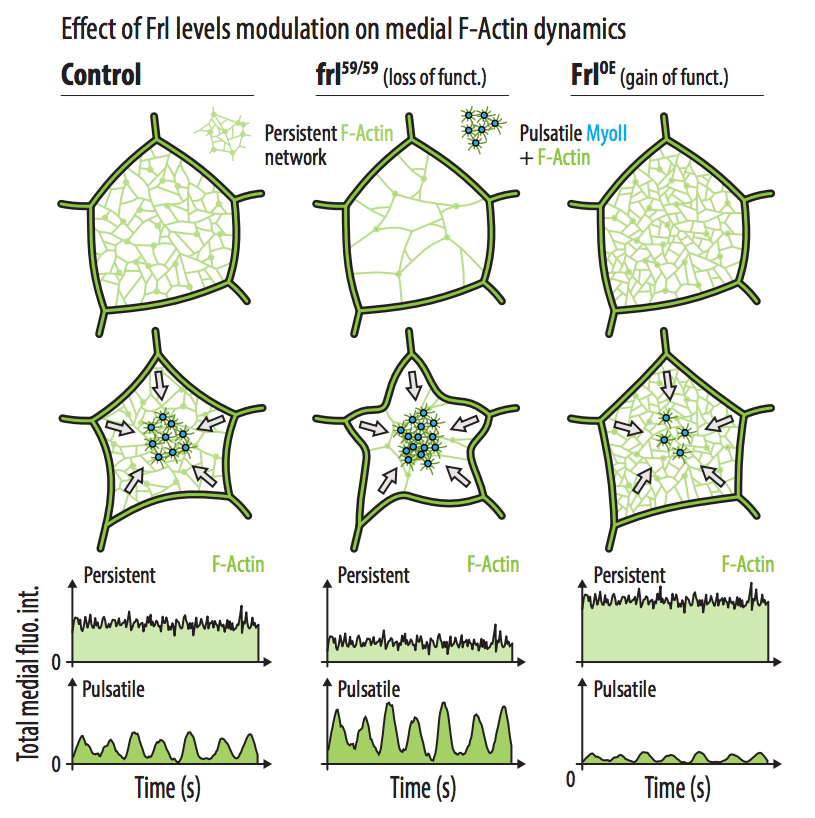
- There are two differentially regulated subpopulations of actin at the medioapical cortex of Drosophila ectodermal cells. In addition to pulsatile F-actin polymerisation occurring in synchrony with the well-known Myosin II pulses, there is also a persistent homogeneous network of actin filaments.
- Unlike the pulsatile F-actin polymerisation, assembly of the persistent actin network does not require RhoA activity.
- The formin Frl/Fmn1 regulates the density of the persistent actin network and counteracts the medial actomyosin pulsatility by modulating Rho activity.
- The pulsatile actomyosin drives cell deformations and the persistent actin network supports the transmission of these contractile forces to cell junctions, enabling their propagation throughout the tissue.
- Disrupting the persistent actin network reduces the propagation range of contractile forces and results in tissue-scale defects in germband extension and dorsal closure.
Why I chose this preprint & how it moves the field forward
The molecular and mechanical details of actomyosin networks can sometimes be heavy reading for the non-specialist but this paper is beautifully written. The authors proceed logically through the experiments explaining the rationale behind each one and allowing the reader to follow their thinking. The experiments themselves are often relatively simple but the imaging is excellent and the analysis is elegant. The use of nicely designed schematics also helps to explain the findings at a glance.
Importantly, the authors’ approach allows them to look beyond the pulsatile myosin contractility that has been well studied during morphogenesis, and begin to tease apart the effects of the organisation of the cortical actin network. This is interesting because it provides new insights into an additional level of actin-mediated regulation of epithelial cell dynamics that is likely to be relevant to many other developmental contexts. By differentially modulating the subpopulations of actin filaments, cells may be able to fine tune the degree of cellular deformation and the spatial propagation of forces at different times during development and within different tissues.
More broadly, I think this paper is a wonderful example of how variations in the expression level of a single protein can cause molecular alterations within the cell that alter mechanical properties and have far-reaching consequences for morphogenesis.
Future directions and questions for the authors.
This paper identified a new role for formins distinct from their well-known roles in lamellipodia/filopodia formation. Future work will likely reveal how formin activity is regulated in this context and which specific formins are involved.
The authors showed that the persistent actin network acts to inhibit Rho activity but it remains to be shown how this happens.
Actomyosin pulsing also occurs during development in C.elegans3, Xenopus4 and the preimplantation mouse embryo5. It will be interesting to see whether similar subpopulations of cortical actin filaments exist in these systems and if they also act to tune cell deformation and the propagation of forces across the tissue.
Q1: What motivated you to look for a subpopulation of actin filaments distinct from the contractile network?
Q2: Previous research on apical constriction proposed the existence of a “molecular clutch” that modulates the strength of the connection between adherens junctions and the apical actomyosin network6. Although this is usually thought of in terms of adhesion complex regulation, could Frl/Fmn1 also be thought of as a clutch mechanism by controlling the density of the persistent actin network, and therefore the strength of the connection between actin and the junctions?
Q3: Formins also regulate the microtubule cytoskeleton and microtubules have recently been shown to promote connections between medioapical actomyosin and adherens junctions in Drosophila epithelia7. Did you consider any potential effects of the formin manipulations on the microtubule network?
References
1 Coravos, J. S., Mason, F. M. & Martin, A. C. Actomyosin Pulsing in Tissue Integrity Maintenance during Morphogenesis. Trends Cell Biol 27, 276-283 (2017).
2 Blanchard, G. B., Etienne, J. & Gorfinkiel, N. From pulsatile apicomedial contractility to effective epithelial mechanics. Current opinion in genetics & development 51, 78-87 (2018).
3 Munro, E., Nance, J. & Priess, J. R. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Developmental cell 7, 413-424 (2004).
4 Skoglund, P., Rolo, A., Chen, X., Gumbiner, B. M. & Keller, R. Convergence and extension at gastrulation require a myosin IIB-dependent cortical actin network. Development 135, 2435-2444, (2008).
5 Maitre, J. L., Niwayama, R., Turlier, H., Nedelec, F. & Hiiragi, T. Pulsatile cell-autonomous contractility drives compaction in the mouse embryo. Nature cell biology 17 (2015).
6 Roh-Johnson, M. et al. Triggering a cell shape change by exploiting preexisting actomyosin contractions. Science 335 (2012).
7 Ko, C. S., Tserunyan, V. & Martin, A. C. Microtubules promote intercellular contractile force transmission during tissue folding. The Journal of cell biology (2019).
doi: https://doi.org/10.1242/prelights.12373
Read preprintHave your say
Sign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Deletion of PIEZO1 in adult cardiomyocytes accelerates cardiac aging and causes premature death
Theodora Stougiannou
Megakaryocytes assemble a three-dimensional cage of extracellular matrix that controls their maturation and anchoring to the vascular niche
Simon Cleary
Fis1 is required for the development of the dendritic mitochondrial network in pyramidal cortical neurons
Felipe Del Valle Batalla
Also in the developmental biology category:
Ectopic head regeneration after nervous system ablation in a sea anemone
Isabella Cisneros
Hyaluronic Acid and Emergent Tissue Mechanics Orchestrate Digit Tip Regeneration
Jonathan Townson
Visually-guided compensation of deafening-induced song deterioration
Maitri Manjunath
preLists in the cell biology category:
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Jonathan Townson, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
December in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cell cycle and division 2) cell migration and cytoskeleton 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Matthew Davies et al. |
November in preprints – the CellBio edition
This is the first community-driven preList! A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. Categories include: 1) cancer cell biology 2) cell cycle and division 3) cell migration and cytoskeleton 4) cell organelles and organisation 5) cell signalling and mechanosensing 6) genetics/gene expression
| List by | Felipe Del Valle Batalla et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |
Also in the developmental biology category:
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Jonathan Townson, Jonathan Townson |
BSDB/GenSoc Spring Meeting 2024
A list of preprints highlighted at the British Society for Developmental Biology and Genetics Society joint Spring meeting 2024 at Warwick, UK.
| List by | Joyce Yu, Katherine Brown |
GfE/ DSDB meeting 2024
This preList highlights the preprints discussed at the 2024 joint German and Dutch developmental biology societies meeting that took place in March 2024 in Osnabrück, Germany.
| List by | Joyce Yu |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
2nd Conference of the Visegrád Group Society for Developmental Biology
Preprints from the 2nd Conference of the Visegrád Group Society for Developmental Biology (2-5 September, 2021, Szeged, Hungary)
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Society for Developmental Biology 79th Annual Meeting
Preprints at SDB 2020
| List by | Irepan Salvador-Martinez, Martin Estermann |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EDBC Alicante 2019
Preprints presented at the European Developmental Biology Congress (EDBC) in Alicante, October 23-26 2019.
| List by | Sergio Menchero et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |











 (1 votes)
(1 votes)
5 years
Benoit Dehapiot
What were the most important things you improved in your study as a result of peer review?
Since the release of this prelight our work have been published in Nature Cell Biology (https://www.nature.com/articles/s41556-020-0524-x). One of the questions posed by the reviewers opened new perspectives for future research. Indeed, we have been asked: why the increased cell intercalation observed in the frl59/59 mutants does not accelerate the extension of the germband?
This is in short what we answered to the reviewers:
Ectodermal cell intercalation is one of the key events occurring during germband extension. However, it has been shown that intercalation alone cannot account for tissue extension. Indeed, inhibiting cell intercalation, using eve, runt or Toll-2,6,8 mutants, only reduces extension by 40% (Irvine and Wieschaus, 1994 ; Paré et al, 2014). Other forces are required in this process such as those produced by the mesoderm and the posterior midgut (PMG) invagination (Butler et al, 2009 ; Collinet et al, 2015 ; Lye et al, 2015 ; Bailles et al, 2019). Cell intercalation contributes internal stress to extend the ectoderm, but also provide a means to dissipate elastic energy that accumulates due to PMG pulling on the ectoderm (Collinet et al 2015).
Interestingly, the increased cell intercalation observed in the frl59/59 mutant does not seem to affect the overall tissue extension. In the context detailed above, we would argue that the rate of PMG invagination is somewhat dominant over cell intercalation in defining the pace of germband extension once intercalation is taking place. Intercalation is fast enough to dissipate internal stress due to PMG pulling on the ectoderm.
While many mutants are known to inhibit cell intercalation (see above), the frl59/59 mutant is one of the few known experimental conditions in which the opposite can be observed. The latter could prove to be a very useful tool to further documents the importance of internal and external stresses in tuning the rate of tissue extension.
Irvine, K. D. & Wieschaus, E. Cell intercalation during Drosophila germband extension and its regulation by pair-rule segmentation genes. Development 120, 827–841 (1994).
Paré, A. C. et al. A positional Toll receptor code directs convergent extension in Drosophila. Nature 515, 523–527 (2014).
Butler LC, Blanchard GB, Kabla AJ, et al. Cell shape changes indicate a role for extrinsic tensile forces in Drosophila germ-band extension. Nat Cell Biol. 2009;11(7):859‐864.
Collinet, C., Rauzi, M., Lenne, P.-F. & Lecuit, T. Local and tissue-scale forces drive oriented junction growth during tissue extension. Nat. Cell Biol. 17, 1247–1258 (2015).
Lye, C. M. et al. Mechanical Coupling between Endoderm Invagination and Axis Extension in Drosophila. PLOS Biol. 13, e1002292 (2015).
Bailles A, Collinet C, Philippe JM, Lenne PF, Munro E, Lecuit T. Genetic induction and mechanochemical propagation of a morphogenetic wave. Nature. 2019;572(7770):467‐473.