Nuclear myosin VI regulates the spatial organization of mammalian transcription initiation
Posted on: 5 May 2020 , updated on: 7 August 2023
Preprint posted on 22 April 2020
Article now published in Nature Communications at http://dx.doi.org/10.1038/s41467-022-28962-w
Categories: cell biology
Updated 7 August 2023 with a postLight by Jennifer Black
Congratulations to Hari-Gupta and colleagues on their recent publication in Nature Communications (https://doi.org/10.1038/s41467-022-28962-w) entitled “Myosin VI regulates the spatial organisation of mammalian transcription initiation”. In comparison to the preprint article, there are a few notable differences. In particular, the discussion has been streamlined relative to the original. However, the original hypothesis and model proposed by the authors remains consistent and most changes improve the overall clarity of the article.
Some additional experiments have also been added which complement the original findings described in the preprint. For example, the authors investigate how perturbing the organisation of RNAPII in the nucleus affects the cells. To do this, they treat cells with an inhibitor (‘TIP’) which blocks the motor activity of Myosin VI leading to changes in the location of RNAPII in the nucleus. They found that cells treated with TIP show evidence of transcription becoming suppressed. In the published article, this finding is further supported by an assay that tests for how accessible the chromatin is when the cells are treated with TIP (Figure 6B). In Figure 7, the authors have added an additional ChIP experiment (Figure 7E) which confirms that Myosin VI does associate with the promoters of some genes whose expression changes upon TIP treatment and under stress conditions (as evaluated by RNA-seq in the same figure).
As discussed by the authors, myosin VI can be over expressed in some cancers. This study forms a solid foundation for further work into the role(s) of this myosin during transcription in both normal and aberrant cells.
Reference:
Hari-Gupta, Y., Fili, N., dos Santos, Á. et al. Myosin VI regulates the spatial organisation of mammalian transcription initiation. Nat Commun 13, 1346 (2022). https://doi.org/10.1038/s41467-022-28962-w
Background:
In Eukaryotes, RNA polymerase II (RNAPII) converts DNA into messenger RNA (mRNA) during transcription. Information within the mRNA can then be translated into protein molecules for use by the cell. To prevent unwanted changes in gene expression, which could compromise cellular fitness, transcription is controlled at multiple levels including by the proposed spatial organisation of transcription into distinct ‘factories’, which can contain RNAPII and other regulatory factors required to transcribe the DNA template (1). This is thought to support efficient transcription. What is unclear is how these ‘factories’ are established and maintained in mammalian cells. Here, Hari-Gupta and colleagues, using a range of experimental techniques, uncover a role for a motor protein, myosin VI, in transcription ‘factory’ establishment. Myosins are a family of molecular motors which hydrolyse ATP to drive their ‘walking’ like motion across filaments of actin within the cell to transport cellular cargo but only recently, their activities have been linked to transcriptional regulation. Building on prior knowledge (2+3), the authors interrogate the function further discovering myosin VI can act as a molecular anchor for RNAPII, tethering it to transcription ‘factories’.
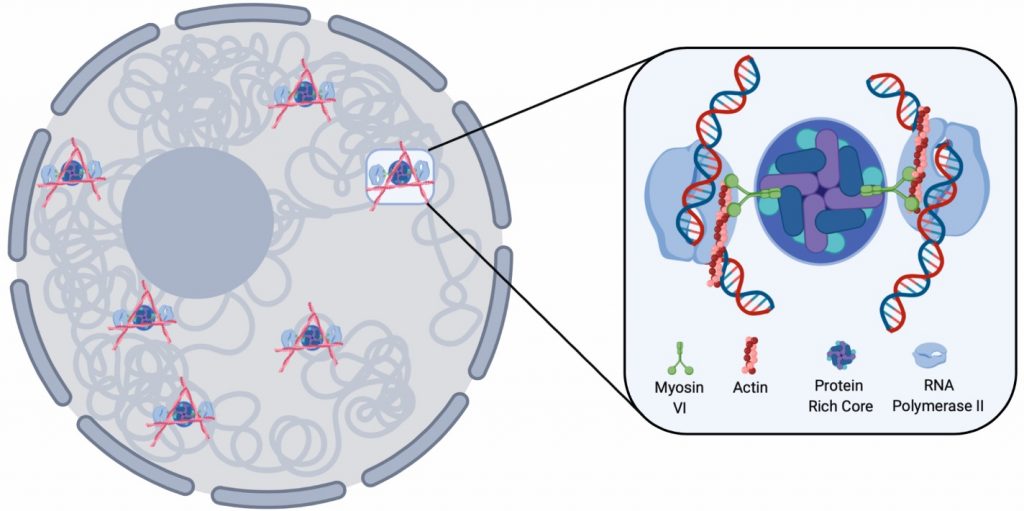
Fig. 1. Diagram depicting the RNAPII anchoring function of myosin VI based on the findings within the paper. Adapted from Figure 9 (CC-BY-NC-ND 4.0 International license)
Key Findings:
- In the nucleus, Myosin VI forms transcription associated clusters
Myosin VI is located in both the cytoplasm and the nucleus of HeLA cells. To study its nuclear localisation in more detail, the authors perform STORM (stochastic optical reconstruction microscopy; 4) super resolution imaging. STORM is a high precision imaging technique using fluorescent probes which alternate between a fluorescing or non-fluorescent state. Instead of capturing all fluorescent signal at the same time, resulting in a more blurred an image, small snapshots are collected, pieced together, and assembled into a final image. Here, the authors reveal ~ 500 clusters of myosin VI present in each nucleus, which increase in number when transcription is stimulated by the addition of serum (~ 700) and in size (from 1.2 to 1.7 um2) but the clusters likely limit the amount of myosin VI molecules within them. They confirm these clusters depend upon the motor activity of the myosin by blocking its activity using the inhibitor TIP [2,4,6-triiodophenol]. By asking if these myosin VI clusters co-localised to transcription initiation regions by detecting RNAPII-pSer5), they find co-localised clusters to be larger (when compared to clustered which did not co-localised) with transcription stimulation increasing cluster number, size and increased co-localisation events between myosin VI and RNAPII-pSer5 (Fig. 1 and 2).
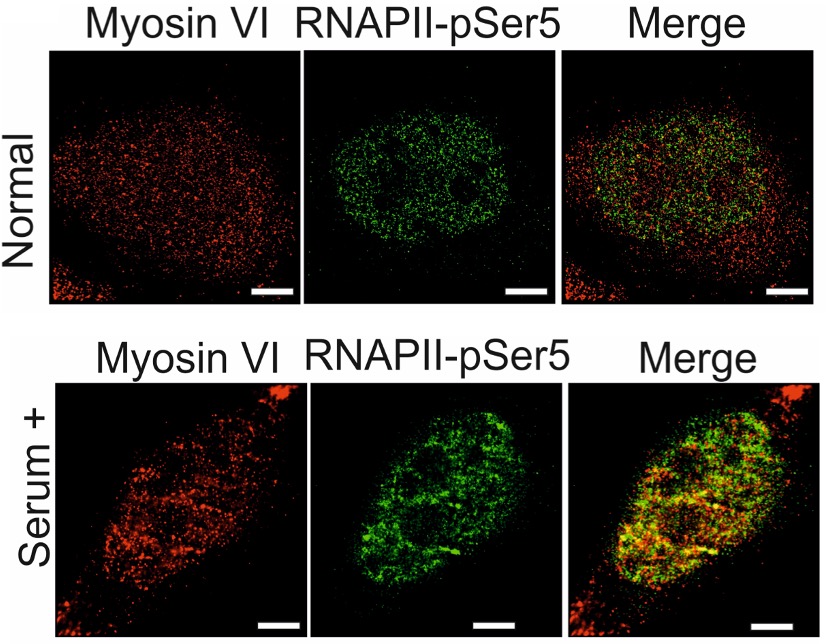
Fig. 2. Images of RNAPII-pSer5 and myosin VI localisation captured using STORM microscopy in the presence and absence of transcriptional stimulation using serum. Adapted from Figure 2 (CC-BY-NC-ND 4.0 International license).
- Myosin VI can organise RNAPII clusters
As shown, myosin VI can organise RNAPII in the nucleus and may direct RNAPII associations with chromatin during transcription initiation. To ask if transcription initiation is disrupted when myosin VI activity is compromised, the authors performed ChIPseq to detect the genomic location of RNAPII-pSer5, in the presence and absence of TIP treatment. After TIP treatment (when compared to untreated cells) they report decreased interactions between RNAPII-pSer5 and the chromatin supporting their hypothesis (Fig. 3). They selectively disrupted the nuclear myosin VI pool with over expressed truncated versions of myosin VI with nuclear localisation signals (NLS) causing nuclear myosin VI to localise aberrantly. Additionally, they confirm this transcription initiation associated RNAPII clustering by myosin VI is actin dependent (Fig. 4).
- The behaviours of Myosin VI and RNAPII are interlinked
Transcription ‘factories’ have proven challenging to image in live cells (1) and here, the authors are unable to visualise RNAPII clustering using single molecule tracking or distinguish between RNAPII-pSer5 and the general pool of RNAPII. However, examining and tracking the movement of all tagged RNAPII (by tagging the subunit Rbp1 with a SNAP or HALO tag), they observed, by stimulating transcription, a slower diffusion of RNAPII molecules in the nucleus (relative to unstimulated conditions). On the other hand, when myosin VI activity was disrupted, either by RNAi or TIP addition or by disrupting actin dynamics, they found RNAPII molecules to be more diffuse (less confined) in the nucleus. By inhibiting RNAPII using alpha-amanitin, the found a similar effect on myosin VI, where the molecules were more diffuse demonstrating that these two proteins can influence the behaviour of each other.
- Myosin VI depletion perturbs transcription
Next, the authors evaluated the effects of myosin VI depletion on global transcription in HeLa cells as addition of TIP reduced active transcription (showing an increase in the repressive chromatin mark H3K9me). RNAi depletion of myosin VI correlated to a 3-fold reduction in growth with the transcriptomic analysis revealing marked effects on processing involving signal regulation, cell communication, proliferation and stimulus responses. Within the down-regulated cohort of genes, two thirds of genes required for the cellular response to serum (used to stimulate transcription) were detected revealing myosin VI is required for gene expression in response to stimuli, in this case the addition of serum.
- Myosin VI can act as a force-induced anchor
Myosin VI can act as an anchor when subject to increased molecular force. As a molecule, RNAPII is large and its potentially mobile behaviour could generate the force needed to induce the anchor-like behaviour of myosin VI. To test this hypothesis, the authors use a ‘molecular spring’ system. Simply, they insert a repeat sequence downstream of its cargo binding domain (Fig. 8) of myosin VI, which does not alter the stability, localisation or activity of this protein. When tension is applied across the ‘spring’ insert, the inserted region unfolds and leaving myosin VI insensitive to changes induced by forces up to 10 pN meaning it should not be able to perform anchoring-like functions. As their earlier results showed, loss of myosin VI disrupts the nuclear localisation of RNAPII-pSer5. Thus, by depleting myosin VI by RNAi and overexpressing the modified ‘spring’ form of myosin VI, the authors found they could not fully restore RNAPII-pSer5 distribution but over expression of the unmodified myosin VI could, supporting their hypothesis that myosin VI plays a role during transcription initiation.
What I liked about this preprint:
I found this preprint very enjoyable to read. The authors use a wide array of techniques ranging from super resolution imaging to genomics datasets and single molecule analysis to examine their hypothesis from different angles to carefully dissect the role of Myosin VI during transcription. Their multi-disciplinary approach adds considerable depth to their findings. Despite the identification of transcriptional ‘factories’ in the 90’s, how they are established has remained largely elusive. The findings in this paper now shed light on this phenomenon and with it, the question of myosin involvement in other types of nuclear cluster formations is raised, for example at regions of active DNA repair.
Questions for the authors:
- You mention using the transcription inhibitor alpha-amanitin affects the diffusion of myosin VI in the nucleus. What happens to the subcellular localisation of myosin VI? Does Myosin VI still form nuclear clusters? Could you ask if myosin VI may act to assist in transcription coupled repair which likely requires similar movement and recruitment of molecular complexes?
- Gene expression can be regulated to respond to different stimuli. Here you use serum to stimulate transcription. Have you examined how myosin VI may behave in the presence of other external stimuli when transcription is stimulated?
- You show that myosin VI can help establish transcriptional ‘factories’ and mention myosin VI can be over expressed in some cancers. As HeLa cells are immortalised, do you think there may be differences between myosin VI’s behaviour in, for example, primary cell lines?
References:
- Buckley M. S., & Lis, J. T. Imaging RNA Polymerase II transcription sites in living cells. Current Opinion in Genetics & Development. 25 (2014)
- Fili, N., Hari-Gupta, Y., Aston, B et al. Competition between two high- and low-affinity protein-binding sites in myosin VI controls its cellular function. J Biol Chem, 295 (2020)
- Fili, N., Hari-Gupta Y., Dos Santos A et al. NDP52 activates nuclear myosin VI to enhance RNA polymerase II transcription. Nat. Comms, 8 (2017).
- Xu, J., Ma, H. & Liu, Y. Stochastic optical reconstruction microscopy (STORM). Curr. Protoc. Cytom. 81 (2017).
doi: https://doi.org/10.1242/prelights.20041
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Resilience to cardiac aging in Greenland shark Somniosus microcephalus
Theodora Stougiannou
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
preLists in the cell biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
December in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cell cycle and division 2) cell migration and cytoskeleton 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Matthew Davies et al. |
November in preprints – the CellBio edition
This is the first community-driven preList! A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. Categories include: 1) cancer cell biology 2) cell cycle and division 3) cell migration and cytoskeleton 4) cell organelles and organisation 5) cell signalling and mechanosensing 6) genetics/gene expression
| List by | Felipe Del Valle Batalla et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |











 (No Ratings Yet)
(No Ratings Yet)