Stem cell-derived mouse embryos develop within an extra-embryonic yolk sac to form anterior brain regions and a beating heart
Posted on: 5 September 2022 , updated on: 19 December 2022
Preprint posted on 2 August 2022
Article now published in at https://www.nature.com/articles/s41586-022-05246-3
Mouse-embryo model derived exclusively from embryonic stem cells undergo neurulation and heart development
Posted on: , updated on: 19 December 2022
Preprint posted on 2 August 2022
Embryonic stem cells, mixed with trophoblast cells and extra-embryonic endodermal cells, make the recipe for a synthetic mouse embryo!
Selected by Monica Tambalo, Juan Moriano, Martin EstermannCategories: cell biology, developmental biology
Updated 1 November 2022 with a postLight by Monica Tambalo
Just a few months after the preprint from Lau and colleagues was posted on bioRxiv it got published in Cell Stem Cell (https://doi.org/10.1016/j.stem.2022.08.013). After comparing the two versions of the manuscript, I did not spot any major differences. The main figures in the published paper are almost identical to the ones in the preprint with just some minor esthetical changes. One difference I could observe is in Figure 4C as part of which – in the published version – a less magnified embryo is shown in the right corner and a few labels were added to help understand the anatomy. The supplementary figures are almost identical between the preprint and the published paper. Now Supplementary Figure 5 has a more detailed analysis of the cellular composition of neurulating embryoids using the single-cell RNA-sequencing data. A few impressive movies have also been included in the published paper showing the beating heart of the synthetic embryos!
The text is very similar when comparing the preprint and published version of this paper and the only real difference is that the latter has a longer discussion section. Just when the preprint was posted on bioRxiv, another group published a similar approach (Tarazi et al., 2022), and related work from the Zernicka-Goetz lab, also discussed in our preLight, was published in Nature (Amadei et al., 2022). The discussion section in the published paper has therefore been extended and comments on these parallel approaches. This also covers the question we asked the authors as part of the preLight about the comparison between the two methods. Lastly, there is a new section covering the limitations of the study, which I really enjoyed reading since it gives a few more details about the successful recipe to generate synthetic mouse embryos.
Amadei, G., Handford, C.E., Qiu, C., De Jonghe, J., Greenfeld, H., Tran, M., Martin, B.K., Chen, D.-Y., Aguilera-castrejon, A., Hanna, J.H., et al. (2022b). Synthetic embryos complete gastrulation to neurulation and organogenesis. Nature 0–1.
Tarazi, S., Aguilera-castrejon, A., Joubran, C., Ghanem, N., Roncato, F., Wildschutz, E., Haddad, M., Gomez-cesar, E., Livnat, N., Viukov, S., et al. (2022). Post-Gastrulation Synthetic Embryos Generated Ex Utero from Mouse Naïve ESCs. Cell.
Background
Soon after the mouse egg is fertilized, successive cell divisions will increase the number of cells exponentially to form a compact mass of cells. Cells in the outer part of this compact mass will become the trophectoderm, while the inner cells will acquire two alternative fates: either primitive endoderm or epiblast. These distinctive specializations will differentially contribute to the development of the future embryo: while the epiblast will be responsible for the generation of the embryo proper, the extra-embryonic structures will be formed by both the trophectoderm (e.g., placenta) and the primitive endoderm (e.g., yolk sac). Around embryonic day 6.5 (E6.5) in the mouse, a process of finely orchestrated rearrangements of epiblast cells, known as gastrulation, begins which results in the formation of the three primary germ layers: ectoderm, mesoderm, and endoderm (Shahbazi and Zernicka-Goetz, 2018). As the basic body plan of the animal is established, specialized tissues and organs next develop from these germ layers. For example, the neural tube is formed through a process called neurulation, where a portion of the ectoderm (the neuroectoderm) folds into a tube at the midline of the embryo, eventually differentiating into specific regions such as the forebrain at the most anterior part or the spinal cord more posteriorly. The endoderm gives rise to the gastrointestinal and respiratory tract, whereas the mesoderm will form the urogenital system and the somites.
In mammals, embryos develop inside the mother’s body, making the study of early embryogenesis a very difficult task. To solve this problem and identify the key mechanisms required for building an embryo, scientists have been trying to develop in vitro systems that can recapitulate the early developmental stages of mammalian embryogenesis. Several stem cell models of the mouse embryo have been generated in the past decade (Harrison et al., 2017; Rivron et al., 2018; Sozen et al., 2018), suggesting a remarkable self-organizing capacity of the early mammalian embryo (Zhu and Zernicka-goetz, 2020). Scientists were also able to generate human blastocysts in vitro from induced pluripotent stem cells (Liu et al., 2021; Sozen et al., 2021). Additionally, gastruloids and trunk-like structures with a neural tube and somites were generated using mouse embryonic stem cells (Beccari et al., 2018; van den Brink et al., 2020; Sozen et al., 2021; Turner et al., 2017; Veenvliet et al., 2020), but these models are unable to recapitulate post-implantation embryogenesis since patterning signals originating form extraembryonic tissues are absent (Shahbazi and Zernicka-Goetz, 2018).
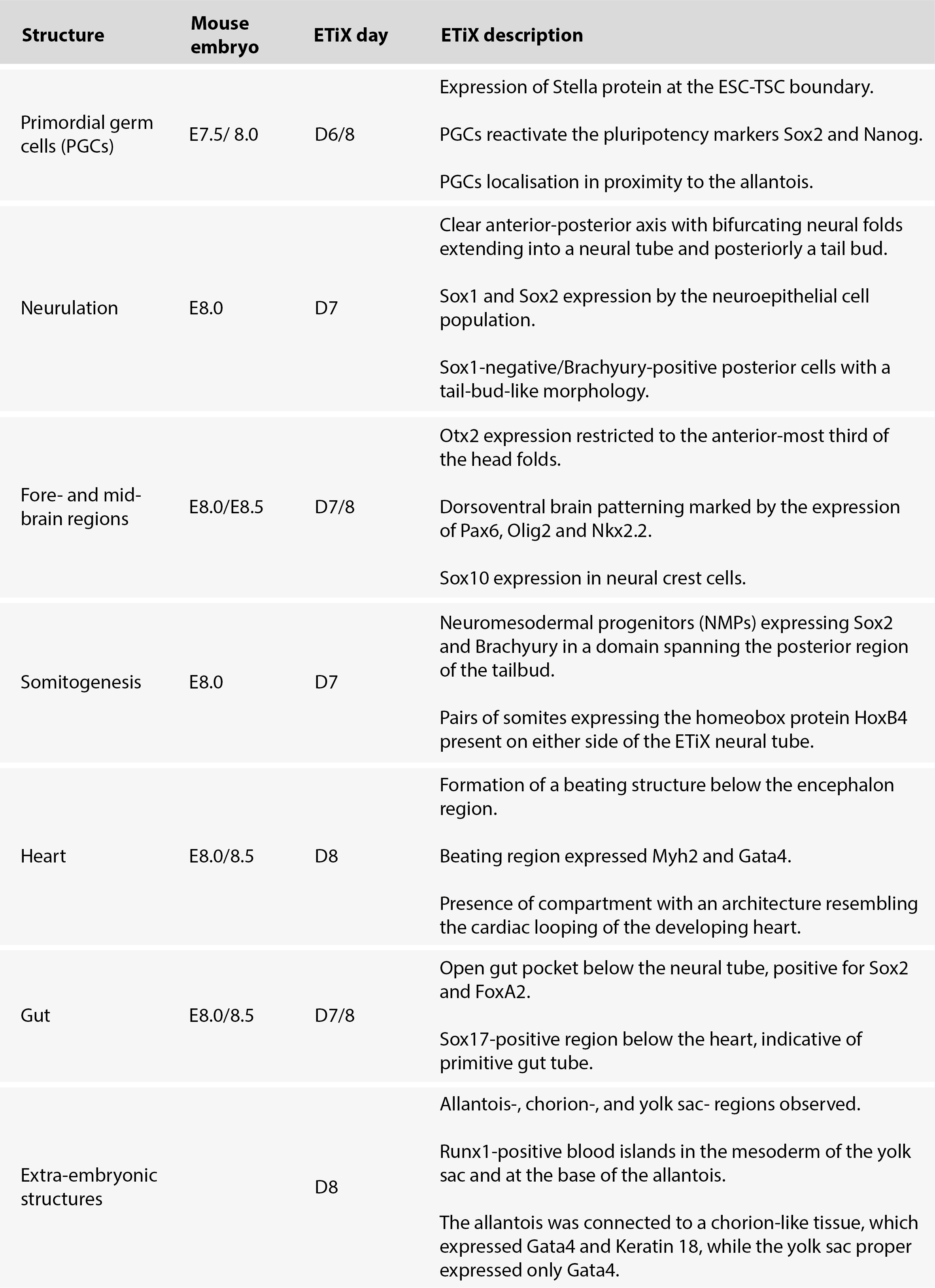
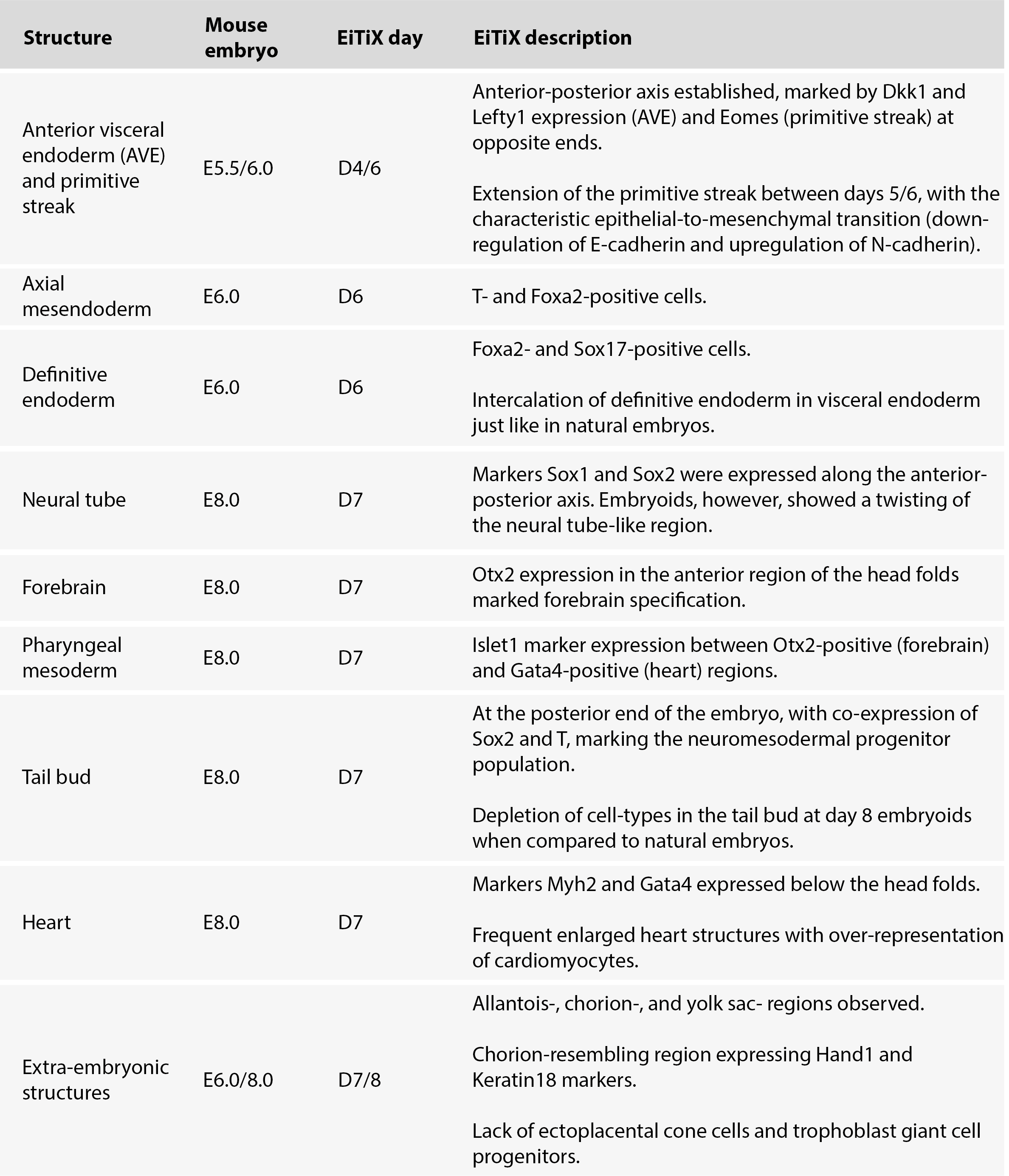
The Zernicka-Goetz Lab has experimented with different recipes to generate a synthetic mouse embryo and has now found two protocols that support the development of a synthetic embryo through post-implantation stages (Amadei et al., 2022a; Lau et al., 2022). The work led by Amadei (Amadei et al., 2022a) has now been published in Nature (Amadei et al., 2022b). In these two preprints, they describe how they were able to generate in vitro mouse embryoids, using two different approaches, that resemble E8.5 natural mouse embryos. These post-implantation-like embryos went through neurulation, developing forebrain and midbrain, produced pairs of somites, developed a beating heart, and initiated gut development. All this is remarkably similar to the development of a natural mouse embryo (Table 1,2).
Main findings
The ETiX-embryoids
In the first preprint from the Zernicka-Goetz Lab (Amadei et al., 2022a), synthetic embryos were generated by aggregating (1) embryonic stem cells (ESCs) from the epiblast, with (2) trophoblast stem cells (TSC; derived from the extraembryonic ectoderm) and (3) extraembryonic endodermal cells. These last ones were differentiated from ESCs by transiently inducing the expression of the extraembryonic visceral endoderm master regulator Gata4. These ETiX embryos developed structures that are remarkably similar to the ones observed in natural mouse embryos from E7.5 to E8.5 (Table 1).
Table 1. Summary table with the main features of the ETiX embryo
The EiTiX-embryoids
In a second preprint, the members of the Zernicka-Goetz Lab aimed to reconstruct mouse early development by solely using embryonic stem cells and transcription factor-mediated reprogramming (Lau et al., 2022). Synthetic mouse embryos, here named EiTiX-embryoids, were generated aggregating: (1) mESCs for epiblast formation; (2) mESCs with inducible Gata4 expression for extraembryonic endoderm formation; and (3) mESCs with inducible Cdx2 expression for trophectoderm formation. This new culture system using mouse embryonic stem cells simplifies the culture conditions since the three cell types use the same culture media.
Table 2. Summary table with the main features of the EiTiX embryo
Of note, a similar approach has been developed by Tarazi and colleagues which was recently published in the journal Cell (Tarazi et al., 2022). Similar to the EiTiX system, their synthetic embryos (sEmbryos) were formed by aggregating non-transduced ESCs with Cdx2-ESCs for the formation of the trophectoderm lineage and Gata4-ESCs to promote primitive endoderm lineage formation. These sEmbryos were grown on an adapted electronic platform which the researchers had previously deployed to grow ex utero mouse embryos for prolonged periods of time (Aguilera-Castrejon et al., 2021).
All these three recent works – ETiX (Amadei et al., 2022a), EiTiX (Lau et al., 2022), as well as the sEmbryos model (Tarazi et al., 2022) – reveal that embryoids recapitulate the development of both embryonic and extraembryonic lineages of the natural mouse embryo, thanks to a detailed analysis of morphology, marker gene expression, and single-cell RNA-seq analyses. Of note, the EiTiX-embryoid model presents some limitations, such as depletion of cell types from the ectoplacental cone lineage, frequently enlarged heart structures, or asynchronicity in cell differentiation. Similarly, sEmbryos presented some differences and/or abnormalities when their development was carefully compared to natural mouse embryos (Tarazi et al., 2022). Nevertheless, these three recipes, even with their current limitations, show that synthetic embryos can adequately complete gastrulation, neurulation, and organogenesis of embryonic and extraembryonic tissues; thus, highlighting the intrinsic capability of naïve pluripotent stem cells to self-organize and give rise to the whole organism in a dish.
Things we like
We are amazed by the striking resemblance of synthetic embryos to natural ones. If one had to blindly assign images to synthetic or natural embryos, it would be a very hard game to play. This resemblance was carefully evaluated by the authors, comparing the embryoids to the natural mouse embryos by immunofluorescence, as well as with a detailed molecular characterization using single-cell RNA-seq. Lastly, the fact that the authors could rescue, to some extent, neural tube defects in the embryoids reveals the great potential of these technologies to model neurodevelopmental disorders and find appropriate treatments.
When the two preprints, along with the Cell paper from the laboratory of Jacob H. Hanna, became available online we immediately thought that they would have a huge impact on the scientific community and beyond. We were right! After just a few weeks, one of the preprints was published in Nature (Amadei et al., 2022b), and the great resemblance of the synthetic embryos to natural ones was quickly picked up by the press (example: https://www.bbc.com/news/health-62679322). Of course, Twitter has also been buzzing with comments and great discussion points.
The peer-reviewed version (Amadei et al., 2022b) of the preprint (Amadei et al., 2022a) that we’ve highlighted here includes some exciting and noteworthy changes. Some that we particularly liked are: the catchier new title, implementations of the histological characterization, more in-depth analysis of the single-cell RNA-sequencing used to compare cell identities in EiTX-embryoids to natural embryos, new experiments evaluating the consequences of Pax6 deletion, and the more elaborate discussion section. We are now looking forward to seeing the second preprint (Lau et al., 2022) appear in a peer-reviewed journal!
Questions for the authors
- What is the proportion of aggregates that successfully become ETiX or EiTiX? Do you have thoughts on how the efficiency could be improved?
- Did you check whether patterning mechanisms (e.g., organizers, signaling centers, the somitogenesis clock) are correctly in place within the ETiX/EiTiX-embryoids?
- Can you speculate on the requirements for longer culture of ETiX/EiTiX-embryoids? How far do you think the system can be pushed?
- Do you think a similar approach could be used for building human synthetic embryoids? What are the ethical restrictions on synthetic embryoids currently in place?
References
Aguilera-Castrejon, A., Oldak, B., Shani, T., Ghanem, N., Itzkovich, C., Slomovich, S., Tarazi, S., Bayerl, J., Chugaeva, V., Ayyash, M., et al. (2021). Ex utero mouse embryogenesis from pre-gastrulation to late organogenesis. Nature 593, 119–124.
Amadei, G., Handford, C.E., De Jonghe, J., Hollfelder, F., Glover, D., and Zernicka-Goetz, M. (2022a). Stem cell-derived mouse embryos develop within an extra-embryonic yolk sac to form anterior brain regions and a beating heart. BioRxiv 2022.08.01.502375.
Amadei, G., Handford, C.E., Qiu, C., De Jonghe, J., Greenfeld, H., Tran, M., Martin, B.K., Chen, D.-Y., Aguilera-castrejon, A., Hanna, J.H., et al. (2022b). Synthetic embryos complete gastrulation to neurulation and organogenesis. Nature 0–1.
Beccari, L., Moris, N., Girgin, M., Turner, D.A., Baillie-Johnson, P., Cossy, A.-C., Lutolf, M.P., Duboule, D., and Arias, A.M. (2018). Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature 562, 272–276.
van den Brink, S.C., Alemany, A., van Batenburg, V., Moris, N., Blotenburg, M., Vivié, J., Baillie-Johnson, P., Nichols, J., Sonnen, K.F., Martinez Arias, A., et al. (2020). Single-cell and spatial transcriptomics reveal somitogenesis in gastruloids. Nature 582, 405–409.
Harrison, S.E., Sozen, B., Christodoulou, N., Kyprianou, C., and Zernicka-Goetz, M. (2017). Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro. Science (80-. ). 356, eaal1810.
Lau, K.Y.C., Rubinstein, H., Gantner, C.W., Amadei, G., Stelzer, Y., and Zernicka-Goetz, M. (2022). Mouse-embryo model derived exclusively from embryonic stem cells undergo neurulation and heart development. BioRxiv 2022.08.01.502371.
Liu, X., Tan, J.P., Schröder, J., Aberkane, A., Ouyang, J.F., Mohenska, M., Lim, S.M., Sun, Y.B.Y., Chen, J., Sun, G., et al. (2021). Modelling human blastocysts by reprogramming fibroblasts into iBlastoids. Nature 591, 627–632.
Rivron, N.C., Frias-Aldeguer, J., Vrij, E.J., Boisset, J.-C., Korving, J., Vivié, J., Truckenmüller, R.K., van Oudenaarden, A., van Blitterswijk, C.A., and Geijsen, N. (2018). Blastocyst-like structures generated solely from stem cells. Nature 557, 106–111.
Shahbazi, M.N., and Zernicka-Goetz, M. (2018). Deconstructing and reconstructing the mouse and human early embryo. Nat. Cell Biol. 22.
Sozen, B., Amadei, G., Cox, A., Wang, R., Na, E., Czukiewska, S., Chappell, L., Voet, T., Michel, G., Jing, N., et al. (2018). Self-assembly of embryonic and two extra-embryonic stem cell types into gastrulating embryo-like structures. Nat. Cell Biol. 20, 979–989.
Sozen, B., Jorgensen, V., Zernicka-Goetz, M., and Chen, S. (2021). Reconstructing aspects of human embryogenesis with pluripotent stem cells. Nat. Commun. 1–13.
Tarazi, S., Aguilera-castrejon, A., Joubran, C., Ghanem, N., Roncato, F., Wildschutz, E., Haddad, M., Gomez-cesar, E., Livnat, N., Viukov, S., et al. (2022). Post-Gastrulation Synthetic Embryos Generated Ex Utero from Mouse Naïve ESCs. Cell.
Turner, D.A., Girgin, M., Alonso-crisostomo, L., Trivedi, V., Baillie-johnson, P., Glodowski, C.R., Hayward, P.C., Steventon, B., Lutolf, M.P., and Arias, A.M. (2017). Anteroposterior polarity and elongation in the absence of extra- embryonic tissues and of spatially localised signalling in gastruloids : mammalian embryonic organoids. Development 3894–3906.
Veenvliet, J. V, Bolondi, A., Kretzmer, H., Haut, L., Scholze-Wittler, M., Schifferl, D., Koch, F., Guignard, L., Kumar, A.S., Pustet, M., et al. (2020). Mouse embryonic stem cells self-organize into trunk-like structures with neural tube and somites. Science (80-. ). 370, eaba4937.
Zhu, M., and Zernicka-goetz, M. (2020). ll Principles of Self-Organization of the Mammalian Embryo. Cell 183, 1467–1478.
How does a single cell generate a complete organism? The concerted changes in shape and function that take place during development, with commonalities and differences among species, and the specification of a rich variety of cell types truly amazed me when I was an undergraduate student. Currently, as a PhD student, it’s exciting to know that we are witnessing remarkable progress in the field of developmental biology with the help of the organoid technology. The works highlighted in this preLights’ post are milestones in the path to model embryonic development with enough fidelity and reproducibility. I was very happy to collaborate with Monica and Martin while writing this post. It wasn’t an easy task to summarize all relevant aspects of the two preprints, but they made the writing easier and smoother. Also, it was enriching to exchange our opinions while writing drafts, where I found we, very often, held similar views.
As a developmental geneticist, it still fascinates me that aggregated cells in culture have the capacity to form complex embryonic structures with such resemblance to in utero grown embryos. As a young postdoctoral researcher switching from birds to mammalian embryonic models, I now understand the difficulties of in utero development when studying embryonic development. These in vitro embryonic models will allow us to monitor and study mammalian embryonic development, under the microscope, in real time!. Particularly, as I study gonadal development and sex determination, which occurs around E10.5-E12.5 in mice, I am interested to see if these models can continue their development in vitro past the E8.5 developmental stage. The preprints and articles discussed here are the starting point of a new era for mammalian developmental biologists. I can’t wait to see where the science takes us.
Have your say
Sign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Resilience to cardiac aging in Greenland shark Somniosus microcephalus
Theodora Stougiannou
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
Also in the developmental biology category:
Cooperation between cortical and cytoplasmic forces shapes planar 4-cell stage embryos
Corentin Mollier, Shivani Dharmadhikari
A drought stress-induced MYB transcription factor regulates pavement cell shape in leaves of European aspen (Populus tremula)
Jeny Jose
Cross Sectional and Longitudinal Imaging Reveals Spatiotemporal Divergence in Morphogenesis and Cell Lineage Specification between in-vivo and in-vitro Mouse Embryo during Pre- and Peri-implantation
Heather Pollington
preLists in the cell biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
December in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cell cycle and division 2) cell migration and cytoskeleton 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Matthew Davies et al. |
November in preprints – the CellBio edition
This is the first community-driven preList! A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. Categories include: 1) cancer cell biology 2) cell cycle and division 3) cell migration and cytoskeleton 4) cell organelles and organisation 5) cell signalling and mechanosensing 6) genetics/gene expression
| List by | Felipe Del Valle Batalla et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |
Also in the developmental biology category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
BSDB/GenSoc Spring Meeting 2024
A list of preprints highlighted at the British Society for Developmental Biology and Genetics Society joint Spring meeting 2024 at Warwick, UK.
| List by | Joyce Yu, Katherine Brown |
GfE/ DSDB meeting 2024
This preList highlights the preprints discussed at the 2024 joint German and Dutch developmental biology societies meeting that took place in March 2024 in Osnabrück, Germany.
| List by | Joyce Yu |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
2nd Conference of the Visegrád Group Society for Developmental Biology
Preprints from the 2nd Conference of the Visegrád Group Society for Developmental Biology (2-5 September, 2021, Szeged, Hungary)
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Society for Developmental Biology 79th Annual Meeting
Preprints at SDB 2020
| List by | Irepan Salvador-Martinez, Martin Estermann |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EDBC Alicante 2019
Preprints presented at the European Developmental Biology Congress (EDBC) in Alicante, October 23-26 2019.
| List by | Sergio Menchero et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |











 (No Ratings Yet)
(No Ratings Yet)
3 years
Monica Tambalo
As a developmental neurobiologist growing brain organoids myself, I was amazed by the complexity of the synthetic embryos generated by these works. Building a ball of progenitors and neurons is now relatively easy with the right protocol, but there is no proper patterning in the majority of current brain organoid protocols (Tambalo and Lodato, 2020). Seeing the synthetic mouse embryos forming with an anterio-posterior axis, a proper neural tube with dorso-ventral patterning, somite pairs, a beating heart, and many other cool features truly amazed me! I personally think that these works will be instrumental for the understanding of the early phases of mammalian embryogenesis. While reading the preprints, I liked how the authors worked on describing the system they have built with a detailed characterization that resembles how developmental biologists study the similarities and differences of embryos from different species. An ethical debate has also arisen since, to our knowledge, there are no current regulations concerning synthetic embryos and the possibility of building similar embryoids starting from human pluripotent stem cells, which is why we’ve asked the authors to comment on this.