Contractile acto-myosin network on nuclear envelope remnants positions human chromosomes for mitosis
Posted on: 9 July 2019 , updated on: 10 July 2019
Preprint posted on 15 March 2019
A little help from my friends: actin and myosin corral mitotic chromosomes so microtubules can find them
Selected by Alyson SmithCategories: cell biology
Why I think this study is interesting
Microtubules are critical to every step of mitosis; without them, chromosomes cannot segregate to the future daughter nuclei. Yet recent studies have hinted at supporting roles for actin and myosin (1-3). This study employs innovative image analysis to show how actin and myosin forces help microtubules capture chromosomes quickly and accurately, highlighting the interdependency of these cytoskeletal systems.
Background
During mitosis, the nuclear envelope disintegrates, allowing mitotic spindle microtubules to capture the chromosomes at their kinetochores (4-6). Spindle microtubules then align the chromosomes at the center of the spindle and separate them to form two identical daughter nuclei.
The spindle microtubules must search for chromosomes in three dimensions in a crowded environment. After the nuclear envelope disintegrates, the chromosomes can access the entire cell, increasing the search volume for spindle microtubules and reducing chromosome capture efficiency. This study aims to understand how cells tackle this problem and ensure timely and accurate chromosome segregation.
To quantify chromosome scattering, the authors measure the chromosome scattering volume (CSV) in live, dividing cells with fluorescently labled DNA. They use 3D images of the fluorescent chromosomes to define the smallest volume that contains all of the chromosomes (the CSV), which allows them to compare chromosome scattering across experimental conditions and timepoints.
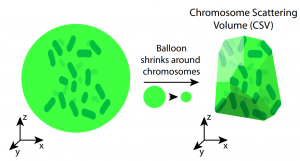
Key findings
Chromosome scatter decreases following nuclear envelope breakdown.
After envelope breakdown, the chromosome scattering volume (CSV) decreased quickly for 8 minutes, then continued to decrease for about 10 more minutes. Overall, the CSV decreased to about 60% of the value before envelope breakdown.
Chromosome condensation does not drive chromosome scatter reduction.
The authors measured total chromosome volume by outlining individual chromosomes instead of the total scatter volume. This value remained constant for 30 minutes after nuclear envelope breakdown, confirming previous findings that chromosome condensation occurs before nuclear envelope breakdown (7).
Microtubules do not drive chromosome scatter reduction.
Treatment with nocodazole to disrupt microtubule polymerization prevented chromosome alignment but did not affect overall CSV reduction. The CSV decreased faster at first in control cells; this could result from fully functional microtubules compressing envelope fragments.
The actin cytoskeleton and the nucleoskeleton coordinate chromosome scatter reduction.
Actin accumulates around the nucleus around 10 minutes prior to nuclear envelope breakdown and surrounds the chromosomes while CSV decreases. High-resolution Airyscan confocal microscopy revealed that the actin network forms just outside the nuclear envelope. The actin-encased volume shrinks to around 65% of its original volume by 6 minutes after envelope breakdown.
The LINC complex links actin in the cytoplasm to the nucleoskeleton. In cells expressing a dominant negative LINC complex, actin failed to accumulate around the nucleus before envelope breakdown. Although chromosome scatter decreased normally for around 8 minutes, reduction then tapered off. The CSV ultimately decreased half as much as in control cells.
Myosin II contractility reduces chromosome scatter.
Myosin II co-localizes with actin at the nuclear envelope prior to its breakdown. Just as with expressing dominant negative LINC, inhibiting myosin II motor activity with blebbistatin stops actin network accumulation/contraction and chromosome scatter reduction. To confirm the role of myosin, the authors used azidoblebbistatin, which covalently links to myosin upon exposure to infrared light. When they exposed one half of the nucleus to infrared light (inhibiting myosin), it did not contract, while the half of the nucleus did.
Actomyosin-driven chromosome scatter reduction ensures proper anaphase onset.
In cells expressing the dominant-negative LINC complex, the mitotic spindle microtubules assembled correctly but there was a considerable delay in chromosome congression (30 vs 64 minutes) and anaphase onset (40 vs 78 minutes). Many cells also had chromosomes behind the spindle poles, which further delayed anaphase.
Future directions
The authors speculate that nuclear envelope breakdown reduces tension in the nuclear membrane (and the associated actin network), which may induce contraction of the envelope-associated actomyosin network to facilitate chromosome scatter reduction. Previous work has shown connections between cell membrane tension and actomyosin contractility (8, 9). Future studies could investigate how changes in nuclear membrane tension affect chromosome scatter reduction during mitosis (10).
How are the mechanisms described in this paper conserved across different cell types? The U2OS cells used in this study are nearly triploid. The authors found an actin network around the nucleus in dividing diploid RPE cells but found no similar network in highly aneuploid HeLa cells. Future work could investigate the relationship between actomyosin-induced chromosome scatter reduction, aneuploidy, and cancer.
References
1. P. Lenart et al., A contractile nuclear actin network drives chromosome congression in oocytes. Nature 436, 812-818 (2005).
2. M. Burdyniuk, A. Callegari, M. Mori, F. Nédélec, P. Lénárt, F-Actin nucleated onchromosomes coordinates their capture by microtubules in oocyte meiosis. The Journal of Cell Biology 217, 2661-2674 (2018).
3. B. Mogessie, M. Schuh, Actin protects mammalian eggs against chromosome segregation errors. Science 357, (2017).
4. T. U. Tanaka, Kinetochore-microtubule interactions: steps towards bi-orientation. The EMBO Journal 29, 4070-4082 (2010).
5. K. M. Godek, L. Kabeche, D. A. Compton, Regulation of kinetochore-microtubule attachments through homeostatic control during mitosis. Nature Reviews. Molecular Cell Biology 16, 57-64 (2015).
6. H. Maiato, A. M. Gomes, F. Sousa, M. Barisic, Mechanisms of Chromosome Congression during Mitosis. Biology 6, 122-177 (2017).
7. F. Mora-Bermudez, D. Gerlich, J. Ellenberg, Maximal chromosome compaction occurs by axial shortening in anaphase and depends on Aurora kinase. Nature Cell Biology 9, 822-831 (2007).
8. B. Pontes, P. Monzo, N. C. Gauthier, Membrane tension: A challenging but universal physical parameter in cell biology. Seminars in Cell & Developmental Biology 71, 30-41 (2017).
9. N. C. Gauthier, M. A. Fardin, P. Roca-Cusachs, M. P. Sheetz, Temporary increase in plasma membrane tension coordinates the activation of exocytosis and contraction during cell spreading. Proceedings of the National Academy of Sciences of the United States of America 108, 14467-14472 (2011).
10. B. Enyedi and P. Niethammer, Nuclear membrane stretch and its role in mechanotransduction. Nucleus 8(2), 156-161 (2017).
doi: https://doi.org/10.1242/prelights.11861
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
Kosmos: An AI Scientist for Autonomous Discovery
Roberto Amadio et al.
preLists in the cell biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
December in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cell cycle and division 2) cell migration and cytoskeleton 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Matthew Davies et al. |
November in preprints – the CellBio edition
This is the first community-driven preList! A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. Categories include: 1) cancer cell biology 2) cell cycle and division 3) cell migration and cytoskeleton 4) cell organelles and organisation 5) cell signalling and mechanosensing 6) genetics/gene expression
| List by | Felipe Del Valle Batalla et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |











 (No Ratings Yet)
(No Ratings Yet)