Nanobody-directed targeting of optogenetic tools to study signaling in the primary cilium
Posted on: 5 May 2020
Preprint posted on 15 April 2020
Article now published in eLife at http://dx.doi.org/10.7554/eLife.57907
Hooked to cilia: a novel nanobody-based tool opens the way for acute manipulation of primary cilia.
Selected by Gustavo AguilarCategories: cell biology, synthetic biology
Background
Cells have developed an extraordinary repertoire of compartments, from cellular sub-domains to organelles, vesicles and membraneless organelles. This way, most cellular functions are restricted in space, ensuring the presence of all the required molecular players and minimising the crosstalk with other cellular processes, among other benefits. When trying to manipulate our favourite cellular activity, either to decipher its function or to exploit its possible benefits, it is critical to consider this spatial component, otherwise we could easily be mistaken in our interpretation.
A paradigmatic case of signalling compartmentalisation is the primary cilium, sometimes referred to as the “cell’s antenna”. This surface projection harbours distinct receptors and downstream signalling components in a Lilliputian reaction volume, permitting highly sensitive and fast signal transduction. Among all the cell’s second messengers, cAMP plays a central role in signal transduction in the cilium, acting downstream of diverse GPCRs. In recent years, the direct observation and manipulation of cAMP has been of major interest to understand the functioning of cilia. Currently, two light-activated enzymes permit both the production and hydrolysis of cAMP: the photo-activated adenylate cyclase (bPAC)1 and the light-activated phosphodiesterase (LAPD)2. Despite the availability of optogenetic tools, restricted manipulation of cAMP to the primary cilia has been impossible, hampering the dissection of its local functions.
The tool
Hansen, Kaiser et al.3 propose a novel way to localize virtually any GFP or mCherry tagged protein to the primary cilium. They envisioned that if tagging of the protein of interest per se did not affect protein function, they could use such moiety to “drag” the protein to the cilium. To do so they made use of nanobodies, which are single-chain protein binders derived from heavy-chain only antibodies. Nanobodies have comparable binding affinity to normal antibodies, which together with its small size and solubility, makes them perfect for intracellular usage4. In their case, a nanobody recognizing GFP or mCherry was fused to a cilium-specific protein scaffold, resulting in the localization of the nanobody in the cilium. Upon co-expression of this nanobody and a GFP- or mCherry-tagged protein the latter will be re-localized to the cilium.
The authors apply this method to localize bPAC and LAPD in the cilium and this way achieve local modulation of cAMP levels.
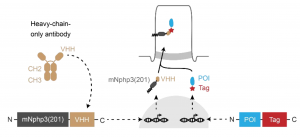
Scheme of the new cilia-targeting approach. Extracted from Figure 2 of Hansen, Kaiser et al. 2020.
The specifics
The authors show that direct fusion of the bPAC and LAPD to protein domains targeted to the primary cilium (in particular the N terminal region of the mouse Nphp3) resulted in ciliary localization but reduced or impeded enzymatic activity.
To overcome this limitation, they developed a bipartite solution to target proteins of interest to the cilium. On one hand, they fused the cilia-specific “scaffold” mentioned before (truncated mNphp3) to an anti-mCherry nanobody, a highly specific protein binder that recognizes the protein mCherry with nanomolar affinity. This cilia-localised nanobody was co-expressed with the mCherry-tagged Optoenzymes bPAC or LAPD, resulting in the latter being “dragged” to the cilia by the nanobody. They confirmed that the nanobody-mediated relocalization of bPAC and LAPD resulted in fully functional enzymatic activity.
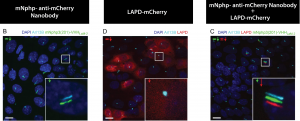
Relocalization of LAPD-mCherry to the cilia. Nanobody in green. LAPD-mCherry in red. Primary cilium in cyan. Dapi in blue. Modified from figure 2 of Hansen, Kaiser et al. 2020.
Next a FRET based reporter of cAMP was targeted to the primary cilia in a similar way. In this case, the authors used an anti-GFP nanobody instead of the anti-mCherry. mNphp3-anti-GFP nanobody fusion could bind the fluorescent proteins of the FRET reporter and localise it to the cilium. The reporter could still detect changes in cAMP concentration.
The new toolkit was then used to investigate the effect of local levels of cAMP in regulating cilia length. Their data supports that local cAMP in the cilium increase its length whereas global cAMP increase is associated with cilia shortening.
Finally, the authors showed that the nanobody-mediated cilia targeting can be used in vivo. Expression of the cilia localised anti-mCherry nanobody in zebrafish could enrich in the cilia an otherwise cytoplasmic RFP.
Why I think this paper is important.
The specific subcellular targeting of functional domains is a challenge with which the authors deal elegantly. Targeting functional domains for long periods may result in unpredictable outputs. In this study, the use of optogenetic tools overcomes this problem, adding temporal control to the setup. In this and few other recent papers5,6,7 the combination of nanobodies and optogenetic tools is starting to flourish and will garner exciting methods for cell biology.
The use of nanobodies against commonly used fluorescent tags allows relocalization of many already existing GFP- and mCherry-tagged proteins to the cilia and will inspire other scientist to tackle the function of the cilia in a much more reliable and imaginative way.
Questions to the authors.
Recently, the list of intracellular protein binders has increased dramatically, Alphatag, Moontag and Frankenbodies are only the tip of the iceberg. These binders recognise small non-fluorescent tags. Do the authors contemplate the use of such binders to be able combine their different toolkit components at the same time? What possibilities would this open?
The validation of the cilia-targeted FRET sensor was done by stimulating the production or hydrolysis of cAMP by drugs. What do the authors think is the sensitivity of the FRET sensor? Would it be able to detect physiological changes in cAMP?
The authors also showed that localisation of bPAC to the cilia at high levels could by itself (without photo-activation) result in a change in cilia length. Could the authors elaborate on this? Does this mean that bPAC has a very low basal activity? Or could it be due to the expression of the mNphp3 scaffold?
The tools seem ready to start exploring when is cAMP produced in the cilia and dissect its role. Apart from control of cilia length, which are the biological questions where the authors think these tools will be useful in the next years?
References.
- Stierl M, Stumpf P, Udwari D, Gueta R, Hagedorn R, Losi A, Gartner W, Petereit L, Efetova M, Schwarzel M, Oertner TG, Nagel G, Hegemann P (2011) Light modulation of cellular cAMP by a small bacterial photoactivated adenylyl cyclase, bPAC, of the soil bacterium Beggiatoa. The Journal of biological chemistry 286: 1181-8
- Stabel R, Stüven B, Hansen JN, Körschen HG, Wachten D, Möglich A (2019) Revisiting and Redesigning Light-Activated Cyclic-Mononucleotide Phosphodiesterases. J Mol Biol 431: 3029-3045
- Hansen, J. N., Kaiser, F., Klausen, C., Stueven, B., Chong, R., Boenigk, W., … & Wachten, D. (2020). Nanobody-directed targeting of optogenetic tools reveals differential regulation of cilia length.BioRxiv.doi: https://doi.org/10.1101/2020.02.04.933440
- Helma, J.; Cardoso, M.C.; Muyldermans, S.; Leonhardt, H. (2015) Nanobodies and recombinant binders in cell biology. Cell. Biol.209, 633–644.
- Deng, W., Bates, J. A., Wei, H., Bartoschek, M. D., Conradt, B., & Leonhardt, H. (2020). Tunable light and drug induced depletion of target proteins. Nature Communications, 11(1), 1-13.
- Yu, D., Lee, H., Hong, J., Jung, H., Jo, Y., Oh, B. H., … & Do Heo, W. (2019). Optogenetic activation of intracellular antibodies for direct modulation of endogenous proteins. Nature methods, 16(11), 1095-1100.
- Gil, A. A., Zhao, E. M., Wilson, M. Z., Goglia, A. G., Carrasco-Lopez, C., Avalos, J. L., & Toettcher, J. E. (2019). Optogenetic control of protein binding using light-switchable nanobodies. BioRxiv, 739201. doi: https://doi.org/10.1101/739201
doi: https://doi.org/10.1242/prelights.20026
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
Kosmos: An AI Scientist for Autonomous Discovery
Roberto Amadio et al.
Also in the synthetic biology category:
Enzymatic bromination of native peptides for late-stage structural diversification via Suzuki-Miyaura coupling
Zhang-He Goh
Enhancer cooperativity can compensate for loss of activity over large genomic distances
Milan Antonovic
Discovery and Validation of Context-Dependent Synthetic Mammalian Promoters
Jessica L. Teo
preLists in the cell biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
December in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cell cycle and division 2) cell migration and cytoskeleton 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Matthew Davies et al. |
November in preprints – the CellBio edition
This is the first community-driven preList! A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. Categories include: 1) cancer cell biology 2) cell cycle and division 3) cell migration and cytoskeleton 4) cell organelles and organisation 5) cell signalling and mechanosensing 6) genetics/gene expression
| List by | Felipe Del Valle Batalla et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |
Also in the synthetic biology category:
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Advances in Drug Delivery
Advances in formulation technology or targeted delivery methods that describe or develop the distribution of small molecules or large macromolecules to specific parts of the body.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)