Human pluripotent stem cell-derived atrioventricular node-like pacemaker cells exhibit biological conduction bridge properties in vitro and in vivo
Posted on: 27 October 2025 , updated on: 29 October 2025
Preprint posted on 4 September 2025
Biological conduction bridges skip a beat. The preprint authors generate AV node-like pacemaker cells from human pluripotent stem cell populations in order to create a biological conduction bridge.
Selected by Theodora StougiannouCategories: bioengineering, cell biology
‘Biological conduction bridges (BioCB) skip a beat’
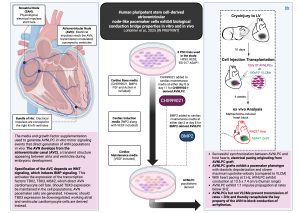
Figure 1. Human pluripotent stem cell-derived atrioventricular node-like pacemaker cells exhibit biological conduction bridge properties in vitro and in vivo’
Background: The atrioventricular node (AVN) is a structure located between the atrial and ventricular heart chambers, found right beneath the endocardium of the right atrium [1]. It receives electrical impulses originating in the atria, usually from the sinoatrial node (SAN) and then allows for these impulses to be transmitted to the ventricles, coordinating contraction of atrial and ventricular chambers. As the fibrous cardiac skeleton is impervious to electrical transmission, the only route of impulse transmission between atria and ventricles is the AVN which in turn connects to other specialized conduction structures located in interventricular tissue, the bundle of His [1]. This separation between atrial and ventricular myocardium is characteristically observed in crocodiles, birds and mammals.
AV block can be described as the delay in, or complete absence of impulse conduction from atria to ventricles; it can be due to structural or functional impairment in AVN function, caused by e.g. various antiarrhythmic substances, ischemic and degenerative disease, as well as by local manipulation in cardiac surgery (during surgical aortic valve replacement, for example [1]).
AV block is usually tackled via implantation of an electronic pacemaker (EPM). The implantation sites employed for pacemaker insertion can include the right ventricular apex (right ventricular pacing [RVP]), a location often associated with intra-ventricular (within ventricles) and inter-ventricular (between ventricles) dyssynchrony, affecting the function of the heart as a pump [2] [3] [4]. While pacing in the right ventricular septum has been proposed as an alternative, as with apex pacing, it can also lead to cardiomyopathy, particularly when ventricular pacing burden exceeds 50% [5]. Pacing in the bundle of His (His bundle pacing [HBP]) is considered to follow physiologic principles of electrical activation in the ventricle, and is described as an alternative to both RVP and biventricular pacing [6]. Though it can improve on the dyssynchrony and associated heart failure hospitalizations, it can be associated with electrical disruptions in the form of P-wave oversensing and R-wave undersensing. Another alternative to the transvenous pacemakers for tackling AV block is leadless pacing (LL), where single- or multicomponent devices are implanted in the myocardium. In the case of multicomponent devices, proper wireless communication between these is required, while battery longevity as well as additional issues around the implantation procedure have also been identified[7]. In short, there is a need to develop biological Conduction Bridges (BioCB) with more favorable biological profiles compared to current implanted pacemaker devices.
During embryonic development cardiac progenitors originate from mesodermal progenitor groups; differentiation protocols thus make use of the signaling required during these stages to generate specific cardiac progenitor groups and cardiac cells in vitro. Ventricular-like cardiomyocytes (VLCM) can be generated with cardiac induction media containing the Wnt signaling inhibitor IWP2 along with VEGF. Atrial-like cardiomyocytes (ALCM) can be generated with cardiac induction media containing IWP2, VEGF along with RA. Sinoatrial-like pacemaker cells (SANLPC) are generated from RALDH2+ mesoderm with WNT inhibition followed by RA, BMP signaling and FGF signaling inhibition. The study by Lohbihler and team seeks to generate AV node-like pacemaker cells from human Pluripotent stem cell (PSC) populations using appropriate signaling modulation. These AVN-like pacemaker cells (AVNLPC) are generated with supplementation of BMP2 after culturing in cardiac induction media used for VLCM derivation [4] [8].
Key aspects of the study:
- AVNLPC can be identified by their expression of TBX3, NKX2-5:
- Activation of WNT signaling via CHIR99021 administered between days 8-11 led to generation of CHIR99021-derived AVNLPC (first detected on day 6).
- Activation of BMP signaling via BMP2 administered between days 5-8 led to the generation of BMP2-derived AVNLPC (first detected on day 6).
- BMP2-derived AVNLPC resemble fetal core AVN pacemaker cells:
- BMP2-derived AVNLPC were evaluated via RNA-sequencing (RNA-seq) (day 20) with GO analysis of biological processes enriched in the TNNT2+ cardiomyocyte cluster linking to processes of regulation of heart contraction, cardiac conduction, cardiac pacemaker differentiation, AV node cell action potential, AVN cell differentiation [4].
- Comparison to RNA-sequencing data of human fetal AVN tissue confirmed that AVNLPCs resemble fetal AVN pacemaker cells.
- AVNLPC generated from the ESI-017-ASAP1 cell line exhibit electrophysiological properties comparable to the AVN in vitro:
- Engineered AVN tissues (eAVNT) exhibited higher rates of diastolic depolarization, slower maximum upstroke potential velocities compered to engineered ventricular tissue; no difference in action potential duration at 30% and 90% of repolarization was observed [4].
- Pacing from the bottom with 2.5Hz led to slow conduction velocities in the eAVNT (3.8±1.5 cm/s) compared to eVT (12.8±2.8cm/s); these slower conduction velocities observed in the eAVNT were comparable to those observed physiologically in humans [4].
- Simulation of fast atrial rhythms by increasing the pacing frequency showed a 1:1 impulse capture ratio by eAVNT until the limit of 3.3±0.2 Hz was reached, a physiological equivalent of 198 beats/min; upon further increase of the pacing frequency, the eAVNT exhibited a conduction block of 2:1 ultimately reaching a ratio of 4:1. No such conduction delay was observed in the eVT engineered tissue, which showed a 1:1 capture ratio up until 4.8±0.3 Hz, a physiological equivalent of 288 beats/min [4].
- eAVNT also exhibited decremental conduction, similar to the endogenous AVN.
- When transplanted into animal models in vivo, AVNLPC exhibit electrophysiological properties comparable to the AVN:
- A guinea pig cryoinjury model was used to create scar tissue and thus, an electrical conduction block in the left ventricular free wall. AVNLPC generated from the ESI-017-ASAP1 or VLCM were then injected into the border zone of the resulting ventricular myocardial scar and hearts were studied 2 to 4 weeks after the injection [4].
- Heart pacing at 2Hz showed synchronization and electrical coupling between AVNLPC or VLCM grafts (ASAP1) and guinea pig hearts (RH237)[4].
- Cessation of heart pacing showed sustained ventricular ectopic beats in all tested animals with AVNLPC grafts, indicating electrical pacing originating from the graft; only 2 out of 7 animals with VLCM exhibited sustained ectopic pacing and the remaining 5 out of 7 animals showed only some sporadic ectopic beats [4].
- AVNLPC grafts exhibited the pacemaker phenotype seen in in vitro experiments, with diastolic depolarization and slower maximum upstroke velocity, when compared with VLCM grafts [4].
- AVNLPC grafts block the conduction of rapid rhythms when transplanted into animal models in vivo:
- Upon heart pacing at 2Hz, AVNLPC grafts displayed conduction in the range of physiological human conduction observed by the AVN, at 13.5±7.4 cm/s; conversely, conduction velocities observed in the VLCM grafts were much higher, at 34.5±14.7 cm/s [4].
- Upon heart pacing at increasing frequencies, AVNLPC grafts showed a 1:1 electrical impulse capture ratio, until the 3.0±0.7 Hz was reached; conversely, VLCM grafts exhibited a 1:1 electrical impulse capture ratio until a higher limit is reached at 6.6±1.4Hz. This highlights the ability of the AVNLPC graft in this study to successfully prevent the transmission of fast atrial rates [4].
- Grafts maintain their molecular identity throughout the entire engraftment period, as shown by the cTNT+ MSX2+ staining observed in AVNLPC grafts and the cTNT+ MSX2low staining observed in VLCM grafts[4].
Why this work is interesting:
The study by Lohbihler and colleagues, carries out a comprehensive evaluation of AVNLPC populations both in vitro and in vivo using a guinea pig model. These cell populations were derived from embryoid bodies (EB) and not adherent monolayer cultures, allowing for the adaptation of these protocols to bioreactor-based protocols and future large scale production [4]. The study also demonstrates the presence of NKX2-5+TBX3+ cardiomyocytes, corresponding to pacemaker cells, derived via standard ventricular cardiomyocyte protocols which can negatively affect cell therapy aimed at treating myocardial infarction. Removal of pacemaker cells by inhibiting BMP signaling after cardiac progenitors are specified can further purify populations meant for myocardial cell therapy, enhancing therapeutic safety [4].
The AVNLPCs derived in this protocol can be used to derive a so-called biological conduction bridge (BioCB) and can be engrafted into the corresponding locations within mammalian hearts to restore conduction in vivo. In the animal model in this study, cells were engrafted via thoracotomy directly onto the injured myocardium (while immunosuppression was also applied to prevent graft rejection) [4]. The engraftment procedure will be easier in larger models, fully ensuring an engraftment area spanning from atria to ventricles; in addition, ensuring these grafts maintain their molecular profile and safe function within human physiological limits is crucial, something which is indeed observed in this study. However, since the AVN is a heterogenous biological structure, the authors acknowledge that further studies evaluating the signaling pathways driving differentiation of each of the cardiomyocyte subtypes identified in the AVN can help generate engineered tissue more closely resembling native AVN structures [4].
Authors one sentence statement on impact of the study:
“This is the first study generating human pluripotent stem-cell derived AVN-like pacemaker cells and demonstrating their functionality for biological conduction bridge applications in vivo”.
Glossary of interesting terms used in this preLight:
- Sinoatrial node (SAN): Characterised as the dominant pacemaker in the heart, found between the superior vena cava and the right atrium [9].
- Atrioventricular node (AVN): Conducts electrical impulses from the atria to the ventricles, an interatrial structure located beneath the endocardium of the right ventricle [1].
- Ventricular conduction system (VCS): Comprises the bundle of His, the left and right bundle branches and lastly, the Purkinje fibers; coordinates contraction of the right and left ventricles with the atria [9].
- Atrioventricular canal (AVC): Transient structure appearing during development, separating developing atria and ventricular tissue.
- Pluripotent stem cells (PSC): Stem cells with the capability to differentiate towards all 3 germ layers of the developing embryo (endoderm, mesoderm, ectoderm) as well as extraembryonic tissues (hypoblast, extraembryonic or primitive endoderm, extraembryonic mesoderm)
- CHIR99021: Small molecule used to induce the WNT signaling pathway; inhibits Glycogen synthase kinase β (GSKβ), thus preventing the phosphorylation and degradation of β-catenin by GSKβ.
- Inhibitor WNT production-2 (IWP2): Small molecule used to inhibit WNT signaling, often used to induce cardiac mesoderm from mesoderm progenitors.
- ASAP1: Fluorescent voltage sensor protein based on a voltage-sensitive phosphatase protein; in this protein the phosphatase domain is removed and a GFP is added in the S3-S4 linker of the molecule. It is used to visually track action potentials, as it gives off green fluorescence in response to changes in action potential. In this study, ASAP1 is used to track electrical activity in the cellular grafts.
- RH237: Voltage-sensitive dye, used to track action potentials in cells such as cardiomyocytes; in this study, RH237 is used to track voltage changes in host guinea pig hearts.
References:
[1] Issa ZF, Miller JM, Zipes DP. Chapter 9 – Atrioventricular Conduction Abnormalities. In: Issa ZF, Miller JM, Zipes DP, editors. Clinical Arrhythmology and Electrophysiology: A Companion to Braunwald’s Heart Disease (Second Edition), Philadelphia: W.B. Saunders; 2012, p. 175–93. https://doi.org/10.1016/B978-1-4557-1274-8.00009-9.
[2] Mashali MA, Saad NS, Peczkowski KK, Fanning T, Hare AN, Whitson BA, et al. Mechanical Dyssynchrony of Isolated Left and Right Ventricular Human Myocardium in End-Stage Heart Failure. Circulation: Heart Failure 2023;16:e009871. https://doi.org/10.1161/CIRCHEARTFAILURE.122.009871.
[3] Tops LF, Schalij MJ, Holman ER, van Erven L, van der Wall EE, Bax JJ. Right Ventricular Pacing Can Induce Ventricular Dyssynchrony in Patients With Atrial Fibrillation After Atrioventricular Node Ablation. Journal of the American College of Cardiology 2006;48:1642–8. https://doi.org/10.1016/j.jacc.2006.05.072.
[4] Lohbihler M, Lim AA, Massé S, Kwan M, Mourad O, Mastikhina O, et al. Human pluripotent stem cell-derived atrioventricular node-like pacemaker cells exhibit biological conduction bridge properties in vitro and in vivo 2025:2025.09.04.674322. https://doi.org/10.1101/2025.09.04.674322.
[5] Biffi M, Bagatin A, Spadotto A, Lazzeri M, Carecci A, Bartoli L, et al. Atrioventricular Block Treatment: Pacing Site, AV Synchrony, or Both? Journal of Clinical Medicine 2025;14:980. https://doi.org/10.3390/jcm14030980.
[6] Lewis AJM, Foley P, Whinnett Z, Keene D, Chandrasekaran B. His Bundle Pacing: A New Strategy for Physiological Ventricular Activation. Journal of the American Heart Association 2019;8:e010972. https://doi.org/10.1161/JAHA.118.010972.
[7] Lee JZ, Mulpuru SK, Shen WK. Leadless pacemaker: Performance and complications. Trends in Cardiovascular Medicine 2018;28:130–41. https://doi.org/10.1016/j.tcm.2017.08.001.
[8] Kim M-S, Horst A, Blinka S, Stamm K, Mahnke D, Schuman J, et al. Activin-A and Bmp4 Levels Modulate Cell Type Specification during CHIR-Induced Cardiomyogenesis. PLOS ONE 2015;10:e0118670. https://doi.org/10.1371/journal.pone.0118670.
[9] Park DS, Fishman GI. 29 – Cell Biology of the Specialized Cardiac Conduction System. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside (Sixth Edition), Philadelphia: W.B. Saunders; 2014, p. 287–96. https://doi.org/10.1016/B978-1-4557-2856-5.00029-7.
doi: https://doi.org/10.1242/prelights.41818
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the bioengineering category:
A Novel Chimeric Antigen Receptor (CAR) - Strategy to Target EGFRVIII-Mutated Glioblastoma Cells via Macrophages
Dina Kabbara
Human pluripotent stem cell-derived macrophages modify development of human kidney organoids
Theodora Stougiannou
Matrix viscoelasticity regulates dendritic cell migration and immune priming
Roberto Amadio
Also in the cell biology category:
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
Kosmos: An AI Scientist for Autonomous Discovery
Roberto Amadio et al.
preLists in the bioengineering category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Advances in microscopy
This preList highlights exciting unpublished preprint articles describing advances in microscopy with a focus on light-sheet microscopy.
| List by | Stephan Daetwyler |
Also in the cell biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
December in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cell cycle and division 2) cell migration and cytoskeleton 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Matthew Davies et al. |
November in preprints – the CellBio edition
This is the first community-driven preList! A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. Categories include: 1) cancer cell biology 2) cell cycle and division 3) cell migration and cytoskeleton 4) cell organelles and organisation 5) cell signalling and mechanosensing 6) genetics/gene expression
| List by | Felipe Del Valle Batalla et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |











 (No Ratings Yet)
(No Ratings Yet)