Mitotic R-loops direct Aurora B kinase to maintain centromeric cohesion
Preprint posted on 15 January 2021 https://www.biorxiv.org/content/10.1101/2021.01.14.426738v1
Categories: cell biology
Background:
During mitosis, chromosomes undergo several structural changes to prepare for sister chromatids segregation into two daughter nuclei. The two sister chromatids are linked by a structure known as a centromere; in humans, this structure is found between the short arm and long arm of the chromosome and it is here that the mitotic spindle attachments occur which facilitate the separation of the two. Recently, RNA-DNA hybrids (R-loops), which are secondary DNA structured composed of an RNA strand attached to an open double-strand of DNA, have been found at centromeres in human cells undergoing mitosis suggesting they may play a role in regulating this process (1). Why they form and their functionality in the centromere is unclear, though they were shown to activate a kinase, Aurora B (AUKB), which has known roles in the regulation of mitosis (2). AUKB is the kinase member of a complex known as the chromosomal passenger complex (CPC), whose other members include INCENP (inner centromere protein), Borealin and Survivin. Though AUKB activities play a role in resolving mitosis associated centromeric R-loops, it is unknown if the rest of the CPC play roles. Here, the authors investigate the timing of R-loop formation during mitosis and asked what factors may act in response to centromeric R-loops. They show that centromeric R-loops remain through prophase but are resolved before anaphase in a manner dependent upon AUKB activity, the CPC and an RNA interacting factor called RBMX.
Key Findings:
- Repetitive regions form R-loops during mitosis
First, the authors asked when and where R-loops form in mitotic cells. They also asked if R-loop formation was affected when the activities of the kinase AUKB were blocked. Using DRIPseq, a modified form of chromatin immunoprecipitation which uses an R-loop specific antibody (S9.6 antibody; 3), the authors stalled cultured human cells in mitosis using a drug called Colcemid then treated the cells with either DMSO (as a control) or one of two AUKB inhibitors (AZD or ZM; 4,5). After collecting and sequencing the DNA from their DRIPseq experiments, they found R-loops form more readily in mitosis at repetitive regions (compared to unsynchronised cells) including alpha satellite regions located at centromeres. By inhibiting the activities of AUKB, they also found R-loops were enrichment at both transcription termination sites and at these alpha satellite regions relative to uninhibited cells. Together, they show repetitive regions accumulate R-loops and AUKB can act to resolve these R-loops.
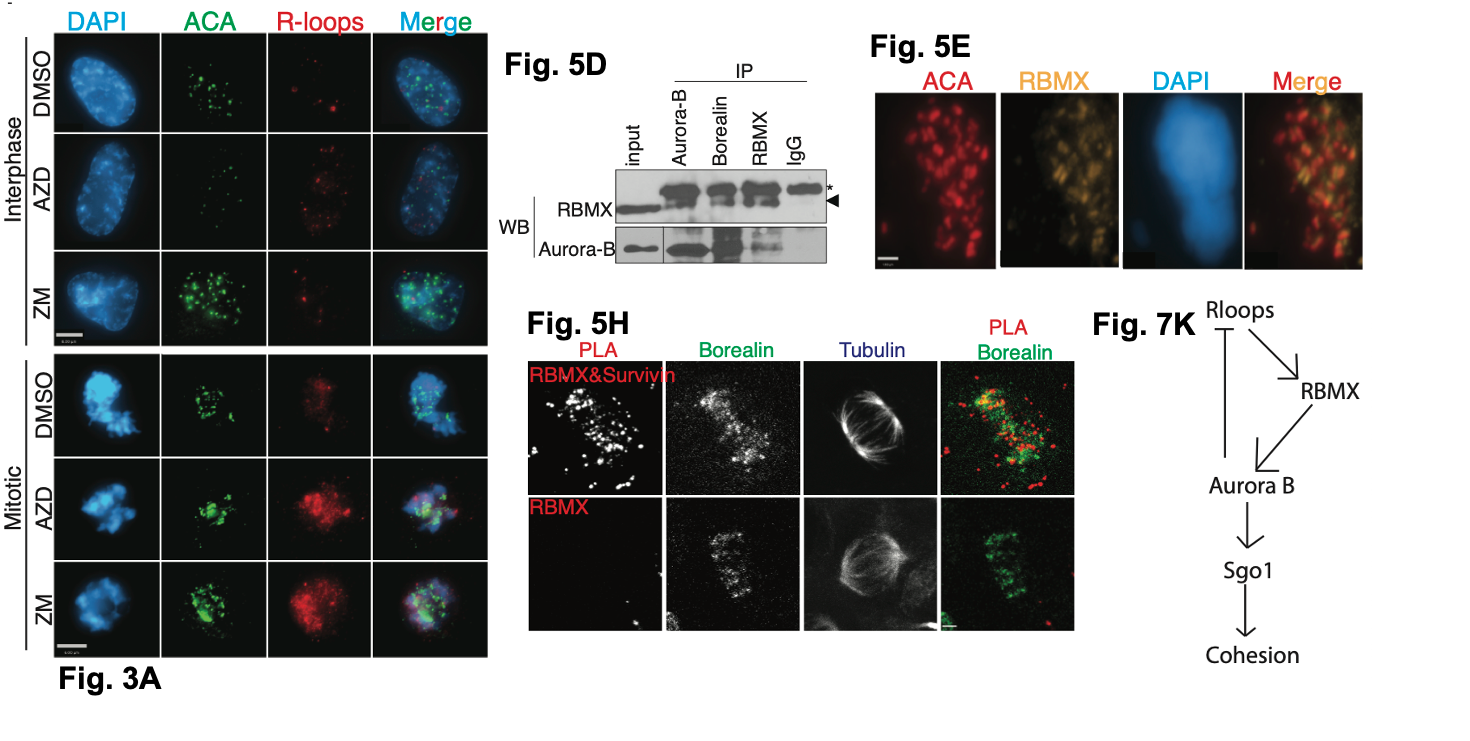
Figure shows selected data from Moran et al. Fig.3A. shows representative images of control (DMSO) or AUKB inhibitor (AZD or ZM) treated cells. Fig.5D shows an immunoprecipitation experiment showing RBMX can interact with the CPC. Fig. 5E shows representative images of the localisation of RBMX to the centromere. ACA is used to mark centromeric location. Fig.5H shows representative images of a proximity ligation assay (PLA) of Survivin (a CPC member) and RBMX. Fig. 7K Schematic summary of the findings from Moran et al. Figures adapted from Moran et al under a CC BY-NC-ND 4.0 licence.
2) R-loops can only be found in centromeres after prophase
Next, the authors asked where R-loops form using immunofluorescence in unsynchronised human cells. To detect R-loops, they used the antibody S9.6. By measuring and quantifying R-loop signal across the chromosomes, they found that R-loop signal was reduced as mitosis progressed, in particular they saw that R-loop formation correlated with chromosome condensation. After prophase, the only R-loops observed were within centromeric regions. Examining the locations of prophase R-loops further via high resolution microscopy, they saw that prophase R-loops formed at sites of heterochromatin (‘silenced’ chromatin) at the nuclear edge. When they correlated R-loop signal with DAPI signal (which stained the chromosomal DNA), the authors found higher R-loops signals were associated with less bright DAPI staining indicating R-loops form when chromosomes condense in early mitosis.
3) AUKB can resolve mitotic R-loops
To ask whether AUKB played a role in centromeric R-loop regulation within mitosis, the authors localised R-loops in unsynchronised cells via microscopy in the presence or absence of AUKB activity. Using two difference AUKB inhibitors, the authors found that inhibiting AUKB activity was associated with higher R-loop levels at chromosomes in mitosis and at their centromeres. They confirmed this using DRIPseq and PCR analysis of these regions. This effect was prominent at alpha satellite repeat regions associated with centromeres (compared with another repeat region). Additionally, depleting R-loops both affected the intensity of AUKB signal by microscopy and the ability of the downstream of AUKB interacting complex (the chromosomal passenger complex; CPC) to drive chromosome movements within the nucleus.
4) CPC acts to regulate centromeric R-loops
To ask if the CPC was directly involved in resolving these centromeric R-loops, the authors purified proteins bound to chromatin in mitotic cells by using CPC factors as bait to capture other bound proteins and a chromatography-based approach (known as Multidimensional Protein Identification Technology; MudPIT; 6) to ask about what proteins were present. They found 111 proteins, including other members of the CPC and known CPC interacting factors such as Topo II alpha. Similar proteins to those found here were also identified when proteins associated with R-loops were purified. The factors identified also implicated both AUKB activity and the downstream CPC complex as key mediators in centromeric R-loop regulation, suggesting these factors can on centromeric R-loops during mitosis.
5) RBMX maintains centromere conhesion via AUKB and CPC activities
One factor that stood out to the authors as a putative regulator of R-loop centromeric activities was the protein RNA binding motif gene, X chromosome (RMBX). RBMX was found to interact with R-loops but also has roles in cohesin at the centromeres and interacts with the CPC. RBMX is enriched on chromatin at alpha satellite regions at the centromere, where it is recruited by the CPC complex. The authors depleted RBMX by shRNA and examined for R-loop and AUKB locations. They found R-loops increased and AUKB localisation to the centromeres was compromised. Their data suggests RBMX is recruited by R-loops to the centromere, where it can recruit AUKB and subsequently the CPC. In the absence of RBMX, cohesion in the centromere was lost; the effect was associated with the inadequate recruitment of a centromeric cohesin regulator called Sgo1. Sgo1 is a factor which acts to maintain centromeric cohesin, stopping the dissociation of cohesin occurring too early in mitosis (7). Sgo1 can be recruited by the CPC and cohesin requires AUKB activity. Together, these data, combined with prior studies indicated centromeric R-loops can bring in RBMX and AUKB, which in turn recruit CPC, followed by Sgo1 to maintain chromatin cohesion prior to division.
In all, the authors have shed light on a novel pathway surrounding the resolution of mitotic R-loops, linking their findings to the activities of a known mitotic regulatory kinase, AUKB, and its associated factors. Their data supports R-loops as key factors for chromosome segregation.
What I liked about this preprint:
I really enjoyed reading this preprint study. Mitotic R-loops were shown recently to have a role at the centromere, but their functions within this discreet region were still uncharacterised. Here, the authors took a combined approach to build upon previous studies and data to ask what mitotic R-loops may be doing. Using high resolution microscopy, known inhibitors and next generation sequencing they were able to propose a model and subsequent pathway for the roles of R-loops at the centromeres during mitosis. I really liked the methodical approach the study took to delineate what events were occurring at the centromere and I found the results very compelling and exciting! Their study raises numerous questions and will open up new routes of investigation in the field of R-loop biology.
Questions for the Authors:
Q1: What are the consequences of failing to resolve R-loops in the centromeric region?
Q2: Regarding the two AUKB inhibitor compounds; why following AZD treatment do you see an increase in R-loops in interphase DNA and at centromeres whereas treatment with ZM did not cause this affect?
Q3: To deplete R-loops, you overexpress RNAseH1. Is it possible that the changes in AUKB localisation you see after the overexpression of RNAseH1 are not solely due to the reduced R-loop levels failing to recruit AUKB but possibly the increased levels of the RNAseH1 enzyme may be interfering with, or even preventing, AUKB recruitment to the centromere?
Q4: The Kabeche et al. study from 2018 suggests centromeric R-loops may be sensed and signalled by the atypical kinase ATR. You suggest RBMX is recruited by R-loops to the centromere. Do you think ATR stimulates its recruitment or are you proposing RBMX is recruited independently to ATR kinase activity?
Q5: What machinery/pathway do think operates to resolve the centromeric R-loops?
References:
- Kabeche, L., Nguyen, H.D., Buisson, R. and Zou, L. A mitosis specific and R-loop driven ATR pathway promotes faithful chromosome segregation. Science (2019).
- Willems, E., Deboddeleer, M., Digregorio, M., Lombard, A., Lumapat, P. N. and Rogister, B. The functional diversity of Aurora kinases: a comprehensive review. Cell Division (2018).
- Ginno, P. A., Lott, P L. Christensen, H. C., Korf, I. and Chedin, F. R-loop formation is a distinctive characteristic of unmethylated human CpG island promoters. Molecular Cell (2012).
- Yang. J., Ikezoe, T., Nishioka, C., Tasaka, T., Taniguchi, A., Kuwayama, Y., Komatsu, N., Bandobashi, K., Togitani, K., Koeffler, H. P., Taguchi, H. and Yokoyama, A. AZD1152, a novel and selective aurora B kinase inhibitor, induces growth arrest, apoptosis, and sensitization for tubulin depolymerizing agent or topoisomerase II inhibitor in human acute leukemia cells in vitro and in vivo. Blood. (2007).
- Walsby. E., Walsh. V., Pepper. C., Burnett. A. and Mills. K. Effects of the aurora kinase inhibitors AZD1152-HQPA and ZM447439 on growth arrest and polyploidy in acute myeloid leukemia cell lines and primary blasts. Haematologica (2008)
- Schirmer, E. C., Yates 3rd, J. R. and Gerace, L. MudPIT: A powerful proteomics tool for discovery. Discovery Medicine (2003).
- Zhang, Q and Liu, H. Functioning mechanisms of Shugoshin-1 in centromeric cohesion during mitosis. Essays Biochem. (2020)
Posted on: 10 February 2021
doi: https://doi.org/10.1242/prelights.27322
Read preprintHave your say
Sign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Clusters of lineage-specific genes are anchored by ZNF274 in repressive perinucleolar compartments
Structural basis of respiratory complexes adaptation to cold temperatures
RIPK3 coordinates RHIM domain-dependent inflammatory transcription in neurons
preLists in the cell biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |











 (No Ratings Yet)
(No Ratings Yet)
3 years
Vasso Episkopou
I admire the depth of this review and the clarity of the answers from the authors.
I wish you good luck