Two redundant ubiquitin-dependent pathways of BRCA1 localization to DNA damage sites
Posted on: 10 August 2021
Preprint posted on 21 July 2021
Article now published in EMBO reports at http://dx.doi.org/10.15252/embr.202153679
The RING sparkle enlightens a new route for BRCA1 recruitment to DNA lesions.
Selected by Giuseppina D'AlessandroCategories: cell biology, molecular biology
Background:
Pathogenic mutations in the BRCA1 gene are associated with an increased risk of developing breast and ovarian cancer (Easton, 1999; Gorodetska 2019). BRCA1 tumor suppressor activity is mainly linked to its function in the repair of DNA double-strand breaks (DSBs), toxic DNA lesions that activate the DNA damage response (DDR), and whose inaccurate repair leads to genome instability and tumorigenesis (Jackson and Bartek, 2009).
The N-terminal RING domain of BRCA1 mediates the interaction with BARD1 and E2-ubiquitin-conjugating enzymes, thus allowing ubiquitylation of target proteins (Figure 1) (Huen, 2010).
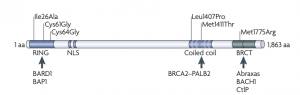
Figure 1. BRCA1 domains architecture. From Huen, M., Sy, S. & Chen, J. Nat Rev Mol Cell Biol, 2010
The C-terminal BRCA1 BRCT domains recognize various phosphorylated DDR proteins, forming distinct BRCA1-containing complexes. Interaction of the BRCT domains with phosphorylated ABRAXAS mediates the formation of the BRCA1-A complex. This complex is thought to recruit BRCA1 to DSBs via RAP80-mediated recognition of the poly-ubiquitin chains deposited by RNF8 and RNF168 (Figure 2).
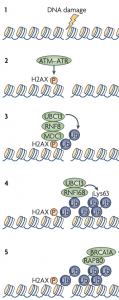
Figure 2. A model for RNF8-/RNF168-mediated ubiquitylation in mediating BRCA1 recruitment to DSBs. Adapted from Huen, M., Sy, S. & Chen, J. Nat Rev Mol Cell Biol, 2010
Key findings:
- BRCA1 is efficiently recruited to DSBs in the absence of RAP80. To investigate the role of chromatin ubiquitylation at DSBs in BRCA1 recruitment, the authors generated knockout (KO) cells for RAP80 by CRISPR/Cas9-mediated genome editing. As expected, these cells fail to form ABRAXAS foci at DSBs induced by ionizing radiation (IR). In contrast, BRCA1 recruitment to DSBs is not affected, while its maintenance is, which suggests that RAP80 (and the BRCA1-A complex) is not necessary for the initial recruitment of BRCA1 to DSBs.
- BRCA1 recruitment to DSBs relies on RAP80 in the absence of RNF168. At DSBs, RAP80 recognizes the K63-ubiquitin chains deposited by RNF8 and RNF168. By using siRNA and KO approaches, the authors show that RNF8 depletion prevents BRCA1 foci formation. However, BRCA1 foci are only reduced by RNF168 depletion and completely lost in the absence of both RNF168 and RAP80. These findings indicate that RAP80-mediated recruitment of BRCA1 becomes essential in the absence of RNF168.
- RAP80-independent BRCA1 recruitment to DSBs occurs via the RING domain. To understand the dependence of RAP80 on BRCA1 recruitment to DSBs, the authors expressed the truncated BRCT domain of BRCA1, whose localization to DSB showed RAP80 dependency. This implies that RAP80-independent recruitment of BRCA1 to DNA lesions must be carried out by a region other than the tandem BRCT domains. To identify this region, the authors expressed various GFP-tagged siRNA-resistant BRCA1 mutants in BRCA1 depleted cells. They observed that two mutants unable to interact with the BRCA1-A complex (via deletion of the BRCT domain or mutation of the ABRAXAS phosphopeptide-binding site) and one mutant consisting only of the RING finger domain are still able to localize to DSBs independently of RAP80. From these observations, they conclude that the RING domain may be important for BRCA1 recruitment redundantly with RAP80.
- RAP80-independent BRCA1 recruitment to DSBs requires interaction with the nucleosome acidic patch or with the E2-ubiquitin ligases via the RING domain. Since mutation or deletion of the RING domain can impair BRCA1 stability, the authors generated BRCA1 constructs harbouring two distinct mutations within the RING domain that abolish the interaction with either the E2-ubiquitin-conjugating enzymes or the nucleosome acidic patch, whilst retaining protein stability. Both mutants accumulate at DSBs in wild-type cells but not in RAP80 KO, implying that both the recognition of the ubiquitylated chromatin via RAP80/BRCA1-A complex and the RING domain are required for BRCA1 recruitment to DSBs. In these mutants, DSB localization of the HR-marker RAD51 also relies on RAP80.
- Inactivation of the RING- and the RAP80-dependent BRCA1 recruitment to DSBs hypersensitizes cells to PARP inhibition. To investigate the clinical relevance of their findings, the authors reconstituted the BRCA1 deficient breast cancer cell line MDA-MB-436 cells with BRCA1 constructs lacking either the BRCT domain or lacking the BRCT domain and harbouring the E2-ubiquitin ligase binding mutation within the RING domain. They found that the double mutant fails to form BRCA1 and RAD51 foci. In line with this observation, cells expressing the double mutant are hyper-sensitive to PARP inhibitor, a drug that kills selectively HR-deficient cancer cells.
Conclusions and future perspectives
In summary, Sherker and colleagues show that chromatin ubiquitylation controls BRCA1 recruitment to DSBs in two ways: one via the BRCT domain-mediated interaction with the BRCA1-A complex (including RAP80) that recognizes K63-ubiquitin chains, the other through the RING domain and dependent on RNF168-mediated ubiquitylation of H2A K13 and K15. Although BARD1 recognizes the H2AK13/K15 ubiquitylation marks (Becker et al., 2021; Dai et al., 2021), the BRCA1 RING mutants used in this preprint (unable to interact with the E2-ubiquitin ligases or with the nucleosomes acidic patch) are still able to interact with BARD1, thus leading the authors to hypothesise alternative mechanisms to explain the observed phenotype.
They propose that BRCA1-mediated H2A ubiquitylation (H2A K125/K127/K129) could promote its own recruitment by favoring SMARCAD1 localization to DSBs and 53BP1 displacement from nucleosomes (Densham et al., 2016). This, in turn, would allow BRCA1-BARD1 to access the H2AK13/K15 ubiquitylated nucleosomes. Alternatively, the interaction with the E2 could be important for positioning BARD1 for the recognition of the H2AK13/15 ubiquitylation.
Since various pathogenic mutations affect the BRCA1 RING domain (Bouwman et al., 2020; Findlay et al., 2018) and tumors expressing RING-less BRCA1 isoforms acquire resistance to therapy, these findings suggest that targeting RAP80, or its interaction with BRCA1, could represent a novel strategy to overcome the resistance of those tumors to DNA damaging agents.
What I liked about this preprint and questions for the authors
I love the idea that BRCA1-mediated H2A ubiquitylation mediates its own recruitment to DSB and it would be very interesting to see what happens after mutating different H2A lysines. Do you believe that other ubiquitylation targets rather than the histones could mediate the observed phenotype?
References:
- Becker, J.R., Clifford, G., Bonnet, C. et al. BARD1 reads H2A lysine 15 ubiquitination to direct homologous recombination. Nature (2021).
- Bouwman, P., van der Heijden, I., van der Gulden, H., de Bruijn, R., Braspenning, M.E., Moghadasi, S., Wessels, L.F.A., Dutch-Belgian, V.U.S.w., Vreeswijk, M.P.G., and Jonkers, J. Functional Categorization of BRCA1 Variants of Uncertain Clinical Significance in Homologous Recombination Repair Complementation Assays. Clin Cancer Res (2020).
- Coleman, K.A., and Greenberg, R.A. The BRCA1-RAP80 complex regulates DNA repair mechanism utilization by restricting end resection. The Journal of biological chemistry (2011).
- Dai, L., Dai, Y., Han, J., Huang, Y., Wang, L., Huang, J., and Zhou, Z. Structural insight into BRCA1-BARD1 complex recruitment to damaged chromatin. Mol Cell (2021).
- Dever, S.M., Golding, S.E., Rosenberg, E., Adams, B.R., Idowu, M.O., Quillin, J.M., Valerie,N., Xu, B., Povirk, L.F., and Valerie, K. Mutations in the BRCT binding site of BRCA1result in hyper-recombination. Aging (2011).
- Densham, R.M., Garvin, A.J., Stone, H.R., Strachan, J., Baldock, R.A., Daza-Martin, M., Fletcher, A., Blair-Reid, S., Beesley, J., Johal, B., et al. Human BRCA1-BARD1 ubiquitin ligase activity counteracts chromatin barriers to DNA resection. Nat Struct Mol Biol (2016).
- Easton, D. F., How many more breast cancer predisposition genes are there? Breast Cancer Research (1999).
- Findlay, G.M., Daza, R.M., Martin, B., Zhang, M.D., Leith, A.P., Gasperini, M., Janizek, J.D.,Huang, X., Starita, L.M., and Shendure, J. Accurate classification of BRCA1 variants with saturation genome editing. Nature (2018).
- Gorodetska, I., Kozeretska, I., Dubrovska, A., BRCA genes: The role in genome stability, cancer stemness and therapy resistance, Journal of Cancer (2019).
- Jackson, S. P. & Bartek, J. The DNA-damage response in human biology and disease. Nature (2009).
- Huen, M., Sy, S. & Chen, J. BRCA1 and its toolbox for the maintenance of genome integrity. Nat Rev Mol Cell Biol (2010).
doi: https://doi.org/10.1242/prelights.30276
Read preprintHave your say
Sign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Resilience to cardiac aging in Greenland shark Somniosus microcephalus
Theodora Stougiannou
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
Also in the molecular biology category:
A drought stress-induced MYB transcription factor regulates pavement cell shape in leaves of European aspen (Populus tremula)
Jeny Jose
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
preLists in the cell biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
December in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cell cycle and division 2) cell migration and cytoskeleton 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Matthew Davies et al. |
November in preprints – the CellBio edition
This is the first community-driven preList! A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. Categories include: 1) cancer cell biology 2) cell cycle and division 3) cell migration and cytoskeleton 4) cell organelles and organisation 5) cell signalling and mechanosensing 6) genetics/gene expression
| List by | Felipe Del Valle Batalla et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |
Also in the molecular biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |











 (No Ratings Yet)
(No Ratings Yet)
5 years
Daniel Durocher
Thank you Giuseppina for highlighting the preprint!
To answer your question regarding potential BRCA1 targets: we are keeping an open mind as to whether H2A is the key BRCA1 substrate, or whether the effect is carried out by another, as-yet undiscovered target. That being said, H2A is the target that makes the most sense to us. Given that the human genome encodes so many H2A genes and isoforms, it is really hard to undertake histone mutagenesis like one could do in budding or fission yeast. Approaches like H2A-Ub fusions may work as it has been used successfully previously. We are still considering what would be the best approach.
However, before jumping ahead we really need to thoroughly test the model that it is indeed the E3 ligase activity that is required for BRCA1 localization in the absence of RAP80!
What is clear is to me is that our work, along with the exciting work from the groups of Ross Chapman, Neil Johnson, Georges Mer, Rachel Klevit, Jun Huang/Zheng Zhou and others opens a new chapter in understand BRCA1 function as a DNA repair factor and we are very excited for what lies ahead!
– Dan