Cell polarity linked to gravity sensing is generated by protein translocation from statoliths to the plasma membrane
Posted on: 4 May 2023 , updated on: 22 November 2023
Preprint posted on 7 April 2023
Amyloplast sedimentation repolarizes LAZYs to achieve gravity sensing in plants
Posted on: , updated on: 22 November 2023
Preprint posted on 18 April 2023
Article now published in Cell at http://dx.doi.org/10.1016/j.cell.2023.09.014
LAZY hitchhikers on statoliths – The missing link between amyloplast movement and auxin redistribution in root gravitropism?
Selected by Marc Somssich, Gwendolyn K. KirschnerCategories: cell biology, developmental biology, plant biology
BACKGROUND:
Amyloplasts – Plant gravisensors
Plants orient themselves along the gravitational vector – the roots grow with it, the shoots against it. After first experiments on this phenomenon were conducted in the 19th century, two seminal papers were published in 1900 which describe a role for non-photosynthetic chloroplasts in the very tip of the root, the columella, as root gravisensors (Haberlandt, 1900; Němec, 1900). These plastids, called amyloplasts due to their starch content, were thus considered statoliths – gravity-sensing organelles.
Auxin – Mediator of gravitropic growth
The next major breakthrough in understanding plant gravitropism was driven by research on the first-described phytohormone, auxin, in the 1920s (Went and Thimann, 1937). Two scientists, Nikolai Cholodny and Frits Went, spearheaded the work on the role of auxin in gravitropism, and eventually it led to the formulation of the Cholodny-Went theory (Cholodny, 1929; as an interesting side-note: Despite this theory being arguably one of the most widely studied and discussed in 20th century plant science, there was never a specific publication dedicated to it. It was simply the result of Cholodny and Went working in parallel on the same topic, while staying in constant exchange and discussion with one another and their peers. Through this constant exchange, the theory just emerged and found its way into the literature). It postulates that growth changes in response to gravity are the result of an unequal distribution of auxin in the growing organ, resulting in different elongation rates on the down- and upward facing side, and thus organ bending. With the advent of plant molecular biology and the availability of several new Arabidopsis thaliana mutants to study gravitropism, the Cholodny-Went theory has been pronounced dead several times due to new, seemingly conflicting results. However, it appears to have even more lives than a cat; because despite all the obituaries published over the past 35 years, its core ideas still hold up pretty well (Sato et al., 2015).
PINs & AUX – Facilitators of auxin-(re)distribution
In the late 1990s, a revival of auxin-research was initiated in part by the description of the first auxin transporters; the PIN efflux, and AUX influx carriers (Gälweiler et al., 1998; Marchant et al., 1999; Sauer and Kleine-Vehn, 2019). Work on the transporters resulted in a model of directional auxin flux within the root, which postulates that shoot-produced auxin is transported through the vasculature toward the root tip. From there, the auxin carriers then redirect auxin from the center toward the periphery of the root and back up toward the shoot. This inverse fountain produces an auxin concentration maximum in the center of the root tip. As a result, the root grows straight down. In response to changes in graviperception, the carriers mediate an unequal redistribution of auxin according to the Cholodny-Went theory, and thus, the root bends toward the ‘new down’.
LAZYs – Bringing it all together?
This now brings up one major question that has occupied the field for years: How do the amyloplasts relay their positional information to the plasma membrane-localized auxin carriers? This is where the LAZY family proteins come in. LAZY mutations were first described in rice and maize in the 1930s, but only in the 1990s, the function of these proteins could be studied in closer detail (van Overbeek, 1936; Jones and Adair, 1938; Nakamura et al., 2019). lazy234 triple mutants are agravitropic, thus they do not respond to changes in the gravitropic vector (Yoshihara and Spalding, 2017). Their amyloplasts still move and relocate according to changes in the vector, but auxin is no longer redistributed upon gravistimulus (Yoshihara and Spalding, 2017; Abe et al., 1994). Thus, these proteins may be involved in the information transfer from amyloplasts to plasma membrane.
MAIN FINDINGS:
In the two preprints discussed here, Nishimura et al. and Chen et al. (2023) have both independently described the principal mechanisms of how LAZY proteins can transfer the gravitropic signal from the amyloplast to the plasma membrane, and thus the compartment in which the auxin carriers can facilitate auxin redistribution.
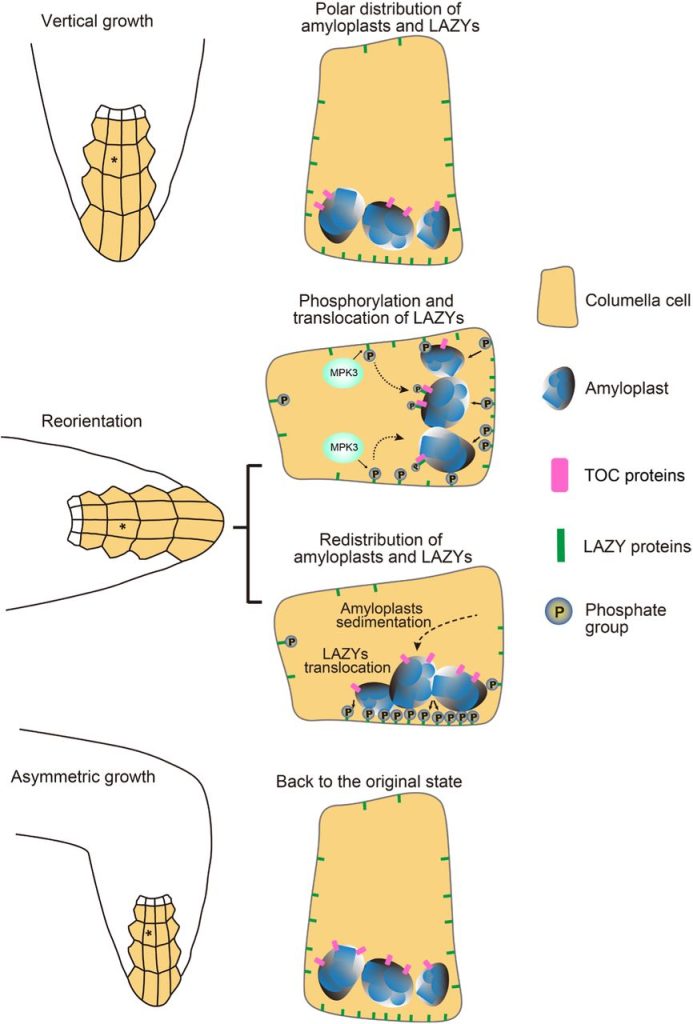
LAZY proteins localize to downward-facing membranes and amyloplasts
Both groups first show that LAZY2, 3 and 4 localize predominantly to the downward-facing plasma membrane of amyloplast-containing columella cells, but more importantly, they also observed them on the outer membrane of the amyloplasts. Since the proteins don’t have any membrane-targeting motifs and sequences, both groups investigated the possibility that hydrophobic regions in the proteins allow them to bind to lipids in the plasma membrane. To this end, Chen et al. used a lipid overlay assay to demonstrate that the LAZYs can indeed bind to phosphatidylinositolphosphates (PIPs). Similarly, Nishimura et al. showed that mutating the hydrophobic regions reduced membrane-localization, indicating that it is these regions that aid in PIP-binding. Furthermore, using a fluorescent biosensor for PIPs, they showed that while these lipids might be involved in localizing the LAZYs to the membrane, they do not function in the relocalization of the proteins following gravistimulation.
Following gravistimulation by a 90° rotation, the LAZY proteins also changed their position from the formerly downward-facing membrane to the newly downward-facing membrane. And this relocalization was dependent on the concurrent relocalization of the amyloplasts, since impaired amyloplastic relocalization in the phosphoglucomutase 1 (pgm) mutant also resulted in impaired LAZY relocalization.
LAZY proteins bind to amyloplasts and move with them to the new downward-facing membrane
Given the localization of LAZYs on the surface of the amyloplasts, both groups speculated that the LAZYs may be literally transported to the membrane by the moving amyloplasts. Nishimura et al. tested this hypothesis using the photoconvertible mEos2 fluorophore. After converting the green fluorescence of amyloplast-localized LAZY4-mEos2 to red, they observed accumulation of red fluorescent LAZY4-mEos2 at the newly downward-facing plasma membrane, indicating that the photoconverted LAZY4s were indeed transferred from the amyloplasts to the plasma membrane. As confirmation, they also employed optical tweezers to trap amyloplasts with photoconverted LAZY4-mEos2, which they then moved into contact with a different membrane. This contact resulted in transfer of photoconverted LAZY4-mEos2 to this new membrane.
LAZYs are bound to amyloplasts by TOC-receptor proteins
Chen et al. used a different approach to show that the LAZYs are indeed hitchhiking with the amyloplasts. They showed that MKK5/MPK3 phosphorylated the LAZY proteins in response to gravistimulation. This phosphorylation induced an interaction of the LAZYs with TRANSLOCON OF OUTER MEMBRANE OF CHLOROPLASTS (TOC) complexes, particularly TOC34, 120 and 132, all of which are receptors on the surface of the amyloplasts. Mutating these TOCs resulted in impairment of LAZY localization to the amyloplasts, relocalization to the plasma membrane, and subsequent auxin redistribution. Thus, the authors concluded that the TOCs bind the LAZYs, once they are phosphorylated in response to gravistimulation, allowing them to move with the amyloplasts to the newly downward-facing membrane (Fig. 1).
SIGNIFICANCE:
While root gravitropism is now a pretty well understood process, and early hypotheses such as the amyloplast-sedimentation and Cholodny-Went models have stood the test of time, there was still a long-standing question: How is the physical stimulus of moving amyloplasts translated into the physiological response of altered auxin distribution (Kawamoto and Morita, 2022)?
The predominant idea of how this could be achieved is mechanotransduction (Häder et al., 2017). With their movement, the starch-filled amyloplasts would put pressure on the actin-cytoskeleton, as well as the membranes of the endoplasmic reticulum and the plasma membrane itself. This force could be perceived by mechanosensitive receptors, which then may indirectly relay the signal to the auxin carriers (Perbal and Driss-Ecole, 2003; Häder et al., 2017). This idea is supported by several experiments and observations, but no such receptor could so far be identified. On the other hand, one very interesting study that used flight-induced weightlessness to investigate this hypothesized role of pressure force in rhizoids of green algae, came to a different conclusion (Limbach et al., 2005). Parabolic flight in a zero-G aircraft or sounding rockets caused microgravity conditions, in which the statoliths were basically weightless. Under these conditions, statolith relocalization to a membrane was still sufficient to induce a gravitropic change in the rhizoids, indicating that contact with the membrane, even without any mechanical pressure, was sufficient for signal transduction (Limbach et al., 2005). With this observation not fitting with the predominant mechanotransduction model, it maybe didn’t receive the attention it should have attracted. However, this observation could now be proven correct, 20 years later, by the results discussed here. With the LAZY proteins being transferred from the surface of the amyloplast to the plasma membrane, contact alone would indeed suffice, and no mechanotransduction would be necessary. Who would have guessed that one of the keys to solve this plant science question would require the deployment of zero-G aircrafts and sounding rockets?
OPEN QUESTIONS:
While the results reported here are a major step forward, there are, of course, still more interesting questions to answer:
One obvious question is what triggers the MKK5/MPK3-mediated phosphorylation of the LAZYs, since this would now pose an early step in the plant’s graviperception.
Then, once the LAZYs arrive at their new destination, how do they relay their signal to the auxin carriers? This is unlikely to be a direct interaction, but this link may be coming by way of RCC1-like domain (RLD) proteins, which co-localize with the LAZYs and may function in PIN-localization (Furutani et al., 2020).
With both LAZY and PIN proteins being localized to phosphatidylinositols and/or membrane nanodomains, it is possible that these proteins are sequestered in membrane domains with distinct lipid signatures (Somssich, 2018; McKenna et al., 2019). In that case, signalling intermediates like RLDs should be present in those same nanodomains, and this could lead to the identification of other proteins involved in this process.
And finally, it will be important to investigate if and how this also affects the gravitropic set-point angle, in which gravitropic responses are not triggered by a certain stimulus but are a constant readout of the gravitropical vector (Kawamoto and Morita, 2022). In lateral roots of Arabidopsis, which grow in a more horizontal angle than the primary roots, this process is at least partially dependent on auxin transport and asymmetric auxin signaling (Rosquete et al., 2013). Therefore, it would be interesting to analyze if the LAZY dependent localization of auxin transporters differs from the primary root, or if it is the readout in later signaling steps that makes the root grow not completely vertically, but at a set angle.
FINAL WORD (Why we chose this preprint):
The root graviresponse is a textbook topic for biology students, and the actual experiment, in which a change in root growth direction can readily be observed within a couple of hours after rotating the plant by 90°, is typically part of basic hands-on plant biology courses. The question of how the tumbling of amyloplasts onto a membrane can trigger a physiological response has thus been a key question discussed in these courses, and we still remember this being the case when we were students 15 and 20 years ago, respectively. To now read a solution to a textbook problem feels like a real milestone, and it is exciting to read about this and preLight it here.
Correction (May 7th, 2023):
In the first version of this article we incorrectly stated that “Nishimura et al. couldn’t detect binding [of LAZY proteins] to lipids”. This has been corrected.
REFERENCES:
Abe, K., TakAhashi, H. and Suge, H. (1994) Graviresponding sites in shoots of normal and “lazy” rice seedlings. Physiol. Plant., 92, 371–374. Available at: https://onlinelibrary.wiley.com/doi/10.1111/j.1399-3054.1994.tb08823.x.
Cholodny, N.G. (1929) Einige Bemerkungen zum Problem der Tropismen. Zeitschrift für wissenschaftliche Biol., 7, 461–481. Available at: https://www.jstor.org/stable/23840913.
Furutani, M., Hirano, Y., Nishimura, T., et al. (2020) Polar recruitment of RLD by LAZY1-like protein during gravity signaling in root branch angle control. Nat. Commun., 11, 76. Available at: http://dx.doi.org/10.1038/s41467-019-13729-7.
Gälweiler, L., Guan, C., Müller, A., Wisman, E., Mendgen, K., Yephremov, A. and Palme, K. (1998) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science (80-. )., 282, 2226–30. Available at: http://www.sciencemag.org/cgi/doi/10.1126/science.282.5397.2226.
Haberlandt, G.J.F. (1900) Ueber die Perception des geotroplschen Reizes. Ber. Dtsch. Bot. Ges., 18, 261–272. Available at: https://www.zobodat.at/publikation_articles.php?id=385590.
Häder, D.-P., Braun, M., Grimm, D. and Hemmersbach, R. (2017) Gravireceptors in eukaryotes—a comparison of case studies on the cellular level. npj Microgravity, 3, 13. Available at: http://dx.doi.org/10.1038/s41526-017-0018-8.
Jones, J.W. and Adair, C.R. (1938) A “lazy” mutation in rice. J. Hered., 29, 315–318. Available at: https://academic.oup.com/jhered/article-lookup/doi/10.1093/oxfordjournals.jhered.a104527.
Kawamoto, N. and Morita, M.T. (2022) Gravity sensing and responses in the coordination of the shoot gravitropic setpoint angle. New Phytol. Available at: https://onlinelibrary.wiley.com/doi/10.1111/nph.18474.
Limbach, C., Hauslage, J., Schäfer, C. and Braun, M. (2005) How to Activate a Plant Gravireceptor. Early Mechanisms of Gravity Sensing Studied in Characean Rhizoids during Parabolic Flights. Plant Physiol., 139, 1030–1040. Available at: https://academic.oup.com/plphys/article/139/2/1030/6113425.
Marchant, A., Kargul, J., May, S.T., Muller, P., Delbarre, A., Perrot-Rechenmann, C. and Bennett, M.J. (1999) AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J., 18, 2066–2073. Available at: http://emboj.embopress.org/cgi/doi/10.1093/emboj/18.8.2066.
McKenna, J.F., Rolfe, D.J., Webb, S.E.D., Tolmie, A.F., Botchway, S.W., Martin-Fernandez, M.L., Hawes, C. and Runions, J. (2019) The cell wall regulates dynamics and size of plasma-membrane nanodomains in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A., 201819077. Available at: http://www.pnas.org/lookup/doi/10.1073/pnas.1819077116.
Nakamura, M., Nishimura, T. and Morita, M.T. (2019) Bridging the gap between amyloplasts and directional auxin transport in plant gravitropism. Curr. Opin. Plant Biol., 52, 54–60. Available at: https://doi.org/10.1016/j.pbi.2019.07.005.
Němec, B.Ř. (1900) Ueber die Art der Wahrnehmung des Schwerkraftreizes bei den Pflanzen. Ber. Dtsch. Bot. Ges., 18, 241–245. Available at: https://www.zobodat.at/publikation_volumes.php?id=57688.
Overbeek, J. van (1936) “Lazy,” an a-geotropic form of Maize. J. Hered., 27, 93–96.
Perbal, G. and Driss-Ecole, D. (2003) Mechanotransduction in gravisensing cells. Trends Plant Sci., 8, 498–504. Available at: https://linkinghub.elsevier.com/retrieve/pii/S136013850300219X.
Rosquete, M.R., Wangenheim, D. von, Marhavý, P., Barbez, E., Stelzer, E.H.K., Benková, E., Maizel, A. and Kleine-Vehn, J. (2013) An Auxin Transport Mechanism Restricts Positive Orthogravitropism in Lateral Roots. Curr. Biol., 23, 817–822. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0960982213003667.
Sato, E.M., Hijazi, H., Bennett, M.J., Vissenberg, K. and Swarup, R. (2015) New insights into root gravitropic signalling. J. Exp. Bot., 66, 2155–2165. Available at: https://academic.oup.com/jxb/article-lookup/doi/10.1093/jxb/eru515.
Sauer, M. and Kleine-Vehn, J. (2019) PIN-FORMED and PIN-LIKES auxin transport facilitators. Development, 146, dev168088. Available at: http://dev.biologists.org/lookup/doi/10.1242/dev.168088.
Somssich, M. (2018) Imaging plasma membrane nanodomains in planta – and how they connect the extracellular cell wall to the intracellular cytoskeleton. preLights, 6238. Available at: https://doi.org/10.1242/prelights.6238.
Went, F.W. and Thimann, K. V. (1937) Phytohormones, New York: The Macmillan Company. Available at: http://www.biodiversitylibrary.org/bibliography/5695.
Yoshihara, T. and Spalding, E.P. (2017) LAZY Genes Mediate the Effects of Gravity on Auxin Gradients and Plant Architecture. Plant Physiol., 175, 959–969. Available at: https://academic.oup.com/plphys/article/175/2/959-969/6116846.
Have your say
Sign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Resilience to cardiac aging in Greenland shark Somniosus microcephalus
Theodora Stougiannou
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
Also in the developmental biology category:
Cooperation between cortical and cytoplasmic forces shapes planar 4-cell stage embryos
Corentin Mollier, Shivani Dharmadhikari
A drought stress-induced MYB transcription factor regulates pavement cell shape in leaves of European aspen (Populus tremula)
Jeny Jose
Cross Sectional and Longitudinal Imaging Reveals Spatiotemporal Divergence in Morphogenesis and Cell Lineage Specification between in-vivo and in-vitro Mouse Embryo during Pre- and Peri-implantation
Heather Pollington
Also in the plant biology category:
A drought stress-induced MYB transcription factor regulates pavement cell shape in leaves of European aspen (Populus tremula)
Jeny Jose
Actin Counters Geometry to Guide Plant Cell Division
Jeny Jose
The nucleus follows an internal cellular scale during polarized root hair cell development
Jeny Jose
preLists in the cell biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
December in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cell cycle and division 2) cell migration and cytoskeleton 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Matthew Davies et al. |
November in preprints – the CellBio edition
This is the first community-driven preList! A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. Categories include: 1) cancer cell biology 2) cell cycle and division 3) cell migration and cytoskeleton 4) cell organelles and organisation 5) cell signalling and mechanosensing 6) genetics/gene expression
| List by | Felipe Del Valle Batalla et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |
Also in the developmental biology category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
BSDB/GenSoc Spring Meeting 2024
A list of preprints highlighted at the British Society for Developmental Biology and Genetics Society joint Spring meeting 2024 at Warwick, UK.
| List by | Joyce Yu, Katherine Brown |
GfE/ DSDB meeting 2024
This preList highlights the preprints discussed at the 2024 joint German and Dutch developmental biology societies meeting that took place in March 2024 in Osnabrück, Germany.
| List by | Joyce Yu |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
2nd Conference of the Visegrád Group Society for Developmental Biology
Preprints from the 2nd Conference of the Visegrád Group Society for Developmental Biology (2-5 September, 2021, Szeged, Hungary)
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Society for Developmental Biology 79th Annual Meeting
Preprints at SDB 2020
| List by | Irepan Salvador-Martinez, Martin Estermann |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EDBC Alicante 2019
Preprints presented at the European Developmental Biology Congress (EDBC) in Alicante, October 23-26 2019.
| List by | Sergio Menchero et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |
Also in the plant biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |











 (4 votes)
(4 votes)
3 years
Hiromasa Shikata
The sentence “While Nishimura et al. couldn’t detect binding to lipids via a fluorescent biosensor…” is misleading. That work just showed that patterns of the biosensors itself do not respond to gravistimulation, while LZYs do following the amyloplast sedimentation. That means the acceptor for LZYs all round on the plasma membrane, which could be an important aspect as the gravity sensor.