Triglyceride metabolism controls inflammation and APOE4-associated disease states in microglia
Posted on: 22 August 2024 , updated on: 7 October 2024
Preprint posted on 13 April 2024
Article now published in Cell Reports at http://dx.doi.org/10.1016/j.celrep.2025.115961
Lipid metabolism is necessary to allow microglial transcriptional and functional changes caused by inflammation and genetic risk factor APOE4 in human iPSC model, making its modulation a possible target to treat various diseases.
Selected by Gustavo Stelzer, Marcus OliveiraCategories: biochemistry, cell biology, neuroscience
Background
Lipid droplets are organelles that essentially store neutral lipids in intracellular compartments, mostly triacylglycerols and sterol esters(1,2). Lipid droplets take part in several cellular functions other than energy storage, such as mounting a response to different types of cellular stress (especially lipotoxic stress due to lipid peroxidation), starvation, oxidation and inflammation(2). Exacerbated accumulation of lipid droplets and their metabolic dysregulation are often linked with a variety of diseases(1).
Apolipoprotein E (APOE) is present in different lipoproteins and mediates their distribution through interactions with plasma membrane receptors(3,4), regulating lipid plasma levels and their transport(3,4). There are three different isoforms, namely APOE2, APOE3 and APOE4. APOE4 is considered one of the main genetic risk factors for sporadic Alzheimer’s disease (AD), expressed in nearly half of AD patients. This isoform has been linked to cognitive impairment, mitochondrial dysfunction(4), neurotoxicity and overall lipid metabolic alterations in different cells, such as neurons and glial cells(3). Because of that, APOE4 is becoming a target to prevent or treat AD(5). Recent papers have demonstrated that APOE4 promotes microglial lipid droplets accumulation(6,7).
Microglia are considered to be the resident immune cells of the brain(8). Being essentially macrophages, microglia are responsible to protect the nervous system against multiple injuries and diseases(8,9). When activated, microglia change their phenotype depending on the stimuli, which include pathogens, amyloid-β peptides, damage-associated molecular patterns and aging(8,10). Frequently, activated microglial cells display pro-inflammatory characteristics, including the production and secretion of cytokines, increased chemokines and enhanced phagocytosis(8,9). Like other macrophages, microglia decrease oxidative metabolism in response to inflammation, which reduces fatty acid oxidation, resulting in the accumulation of intracellular lipid droplets, indicating dysfunctional, reactive and aged microglia(6,7,10). However, the relation between lipid droplets and how they interfere and relate with microglial activation to internal or external stimuli are not well understood yet, which are the main questions authors try to answer in this preprint.
Key findings
LPS-activated microglia display neutral lipid accumulation
Microglia derived from iPSCs carrying the APOE3 genotype exhibited heightened secretion of proinflammatory cytokines and chemokines, increased expression of genes associated with immune activation, enhanced phagocytic activity and morphological changes following LPS stimulation. Transcriptomic analysis revealed an upregulation of genes involved in lipid synthesis and downregulation of genes associated with lipid catabolism upon LPS activation. These transcriptional changes correlated with the intracellular increase of lipid droplets within the microglia.
Triglyceride biosynthesis is necessary for LPS-mediated activation of microglia
To investigate the functional implications of lipid accumulation, the authors pharmacologically inhibited DGAT1 and DGAT2 (involved in fatty acid esterification). Inhibition of these enzymes reduced triglyceride accumulation and altered the transcriptional response to LPS, affecting genes involved in both lipid metabolism and inflammation. DGAT inhibition also decreased NF-κB nuclear translocation and altered chromatin accessibility, indicating downstream effects on inflammatory gene expression. Furthermore, inhibition of triglyceride biosynthesis attenuated microglial amyloid-β phagocytosis in response to the LPS stimulus, suggesting a regulatory role of lipid metabolism in microglial activation and function.
Triglyceride catabolism is necessary for LPS-mediated activation of microglia
Using inhibitors of adipose triglyceride lipase (ATGL) and the phospholipase DDHD2, enzymes involved in triglyceride and phospholipid catabolism, the authors evaluated whether the presence of triglyceride-rich lipid droplets could itself activate microglia. They found that these inhibitors increased lipid droplet accumulation in human iPSC-derived microglia but did not induce cytokine secretion in inactivated cells. However, secretion of multiple cytokines was strongly reduced upon LPS stimulation when ATGL, but not DDHD2, was blocked. This strongly indicates that phospholipid metabolism does not play a role in the microglial response to external activation signals. Moreover, ATGL inhibition also impaired the phagocytosis of amyloid-β following LPS stimulation.
Modulating triglyceride biosynthesis controls the immune state of APOE4 microglia
APOE4 microglia were found to accumulate more lipid droplets than APOE3 microglia even without external LPS stimuli, and both showed increased lipid droplets upon activation. Using DGAT1 and DGAT2 inhibitors, the authors observed that, in unstimulated cells, APOE4 microglia were more affected than APOE3 microglia. DGAT inhibition in APOE4 microglia downregulated genes related to lipid biosynthesis and immune signaling, reducing cytokine expression and secretion, independently of chromatin accessibility and structural changes. Furthermore, DGAT inhibition in both APOE3 and APOE4 microglia normalized disease-associated gene expression patterns.
Graphical abstract
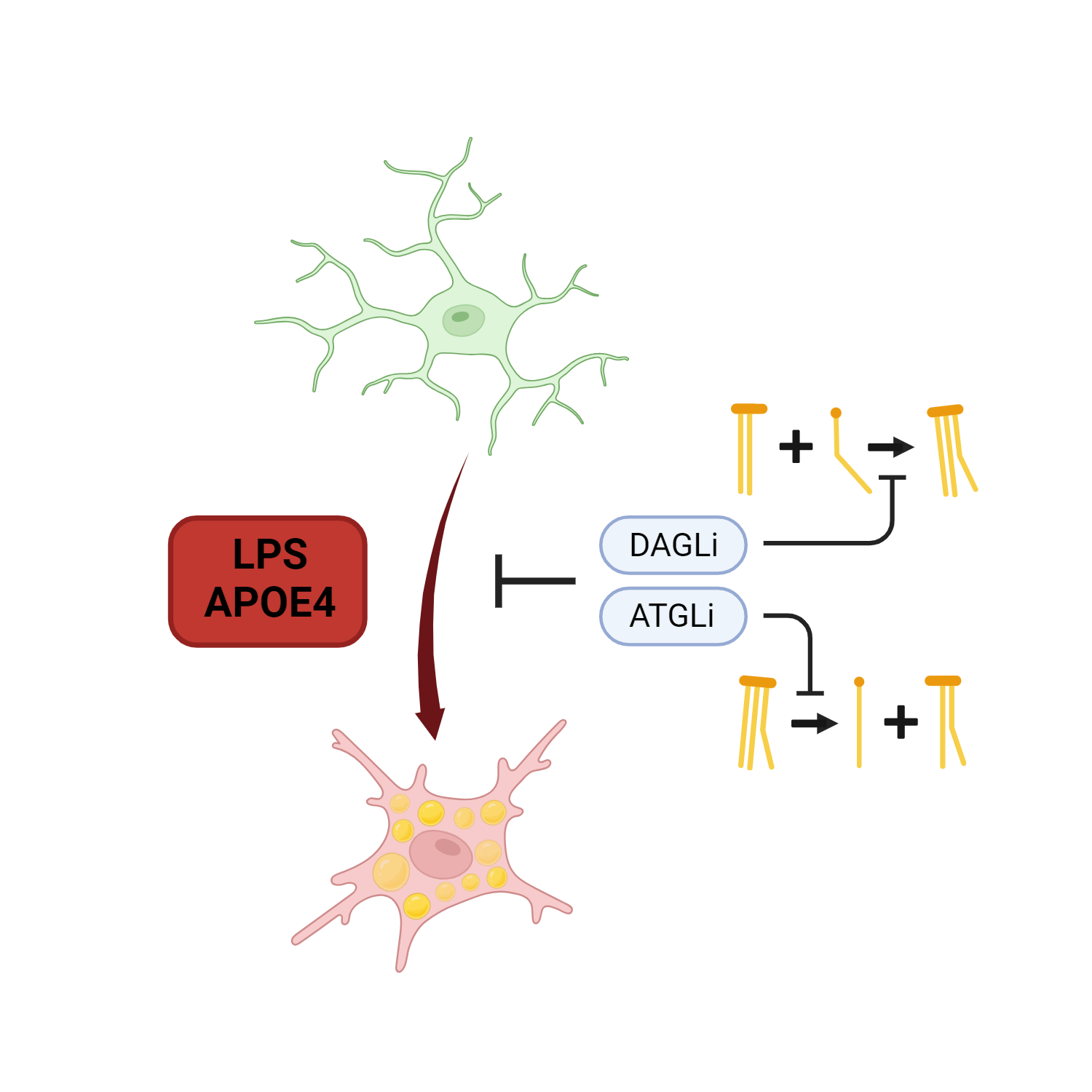
Figure 1: Graphical summary of the Stephenson et al. preprint showing that lipid metabolism participates in phenotypical changes induced by LPS or APOE4 on human iPSC-derived microglia. Image drawn with Biorender.
Why I think this preprint is important
The authors show in an elegant, well-structured and easy-to-understand way that triglyceride metabolism is directly related to microglial activation. The transcriptional and functional alterations induced by LPS stimuli in different human iPSC-derived microglia with different genetic backgrounds appear to be highly dependent on triglyceride biosynthesis and degradation, especially in the APOE4 genotype. These alterations were blocked by inhibitors of enzymes related to fatty acid esterification (DGAT1 and DGAT2) and triglyceride catabolism (ATGL), suggesting that modulation of these pathways could be potentially interesting as a treatment to control neuroinflammation. Through this work, the researchers add more data that strengthen the association between lipid metabolism and inflammation. The authors also open up new possibilities with regard to AD treatment through controlling triglyceride metabolism. Even with its limitations, metabolic pathways modulation is a promising intervention for a myriad of diseases and should be studied in the next years.
Questions and suggestions
Q1: At the end of the first paragraph of the “LPS-activated microglia display neutral lipid accumulation” section there is a typographical error. It now says “CX3XR1” instead of “CX3CR1”.
Q2: The authors could consider standardizing their sample size data presentation. Normally different biological cultures count as different samples and different wells or cells just as experimental replicates. Standardizing this aspect of the paper would increase overall comparability and understanding of the results.
Q3: The authors could mention the techniques used to perform the experiments in figure legends and in the results section. Although the methods were described in the ‘Materials and Methods’ section, I think it would be beneficial for the reader to also get this information while the results are presented.
Q4: The authors could perhaps consider revising some of the data presentation throughout the manuscript. For example, would it be possible to include data from the vehicle, LPS, and LPS + inhibitor groups in the same figures/graphs? Analyzing figures S2 C, J, and L together would for instance provide a clearer picture of the specific effects of LPS activation and DGAT/ATGL inhibition on target genes, helping to evaluate whether these treatments attenuate or completely reverse the alterations caused by LPS. This type of data display would offer a broader understanding of the effects of DGAT/ATGL inhibition.
Q5: Did the authors measure parameters like size, diameter or area of the lipid droplets? According to Benador et al., 2019(11), lipid droplets size and area are related to functional specialization of nearby mitochondria and could suggest different lipid droplet roles. Additionally, size and morphology alterations are related to differences in lipid metabolism in hepatocytes, which determine whether lipid droplets are performing lipolysis or lipophagy(12). One suggestion would be to stain the cells with BODIPY rather than LipidSPOT. This different method would allow the acquisition of confocal lipid droplets images, which is a more effective method to determine lipid droplets size.
Q6: It would be good to specify the conditions in which the cells were harvested (medium, temperature, CO2, etc) in the ‘Materials and Methods’ section.
Q7: Did the authors perform a viability assay on cells stimulated with LPS at 5µgmL? The concentration of LPS used as an external stimulus to promote microglial activation is relatively high and could possibly be inducing cellular death. Additionally, could the authors please explain or reference why they chose this specific stimulation protocol (concentration and duration)? One might argue that microglial cells can be stimulated with lower LPS concentrations and using higher ones might mitigate the fact that vehicle treated cells could already be activated. Either way, I think it would be worth mentioning that inhibitors were able to reverse or attenuate the effects of high LPS concentrations, which shows the importance of lipid metabolism in the inflammatory context in general.
Q8: Since DGAT enzymes are responsible specifically for fatty acid esterification, the authors could consider attributing this functional role other than calling it “lipid biosynthesis”.
Q9: The authors could consider expanding the discussion on why fatty acid esterification and catabolism, apparently antagonistic pathways, have similar effects on microglia. Increased fatty acid esterification and degradation happening simultaneously seems to reflect an involvement of the glycerolipid/free fatty acid cycle, or lipid cycling(13,14). Do the transcriptomic data reveal any further aspects that could support these hypotheses? Indeed, the data on DDHD2 strengthens this conclusion as its activity is not involved in lipid cycling nor in modulating pro-inflammatory markers.
Q10: In order to include the internal stimulus model in the discussion, the authors could perform experiments with ATGL inhibitors in APOE4/APOE4 iPSCs. If the outcomes are similar to those observed with DGAT inhibitors, the authors could suggest that lipid cycling is required to promote microglia activation in both external and internal stimuli. Alternatively, the authors could just include a discussion on lipid cycling in the discussion section in order to explore new aspects of microglial phenotype regulation.
References:
- Zadoorian A, Du X, Yang H. Lipid droplet biogenesis and functions in health and disease. Nat Rev Endocrinol. 2023 Aug;19(8):443-459. doi: 10.1038/s41574-023-00845-0. Epub 2023 May 23. PMID: 37221402; PMCID: PMC10204695.
- Ralhan I, Chang CL, Lippincott-Schwartz J, Ioannou MS. Lipid droplets in the nervous system. J Cell Biol. 2021 Jul 5;220(7):e202102136. doi: 10.1083/jcb.202102136. Epub 2021 Jun 21. PMID: 34152362; PMCID: PMC8222944.
- Huang Y, Mahley RW. Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer’s diseases. Neurobiol Dis. 2014 Dec;72 Pt A:3-12. doi: 10.1016/j.nbd.2014.08.025. Epub 2014 Aug 27. PMID: 25173806; PMCID: PMC4253862.
- Pires M, Rego AC. Apoe4 and Alzheimer’s Disease Pathogenesis-Mitochondrial Deregulation and Targeted Therapeutic Strategies. Int J Mol Sci. 2023 Jan 1;24(1):778. doi: 10.3390/ijms24010778. PMID: 36614219; PMCID: PMC9821307.
- Safieh M, Korczyn AD, Michaelson DM. ApoE4: an emerging therapeutic target for Alzheimer’s disease. BMC Med. 2019 Mar 20;17(1):64. doi: 10.1186/s12916-019-1299-4. PMID: 30890171; PMCID: PMC6425600.
- Sienski G, Narayan P, Bonner JM, Kory N, Boland S, Arczewska AA, Ralvenius WT, Akay L, Lockshin E, He L, Milo B, Graziosi A, Baru V, Lewis CA, Kellis M, Sabatini DM, Tsai LH, Lindquist S. APOE4 disrupts intracellular lipid homeostasis in human iPSC-derived glia. Sci Transl Med. 2021 Mar 3;13(583):eaaz4564. doi: 10.1126/scitranslmed.aaz4564. PMID: 33658354; PMCID: PMC8218593.
- Haney MS, Pálovics R, Munson CN, Long C, Johansson PK, Yip O, Dong W, Rawat E, West E, Schlachetzki JCM, Tsai A, Guldner IH, Lamichhane BS, Smith A, Schaum N, Calcuttawala K, Shin A, Wang YH, Wang C, Koutsodendris N, Serrano GE, Beach TG, Reiman EM, Glass CK, Abu-Remaileh M, Enejder A, Huang Y, Wyss-Coray T. APOE4/4 is linked to damaging lipid droplets in Alzheimer’s disease microglia. Nature. 2024 Apr;628(8006):154-161. doi: 10.1038/s41586-024-07185-7. Epub 2024 Mar 13. PMID: 38480892; PMCID: PMC10990924.
- Hickman S, Izzy S, Sen P, Morsett L, El Khoury J. Microglia in neurodegeneration. Nat Neurosci. 2018 Oct;21(10):1359-1369. doi: 10.1038/s41593-018-0242-x. Epub 2018 Sep 26. PMID: 30258234; PMCID: PMC6817969.
- Abud EM, Ramirez RN, Martinez ES, Healy LM, Nguyen CHH, Newman SA, Yeromin AV, Scarfone VM, Marsh SE, Fimbres C, Caraway CA, Fote GM, Madany AM, Agrawal A, Kayed R, Gylys KH, Cahalan MD, Cummings BJ, Antel JP, Mortazavi A, Carson MJ, Poon WW, Blurton-Jones M. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron. 2017 Apr 19;94(2):278-293.e9. doi: 10.1016/j.neuron.2017.03.042. PMID: 28426964; PMCID: PMC5482419.
- Marschallinger J, Iram T, Zardeneta M, Lee SE, Lehallier B, Haney MS, Pluvinage JV, Mathur V, Hahn O, Morgens DW, Kim J, Tevini J, Felder TK, Wolinski H, Bertozzi CR, Bassik MC, Aigner L, Wyss-Coray T. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat Neurosci. 2020 Feb;23(2):194-208. doi: 10.1038/s41593-019-0566-1. Epub 2020 Jan 20. Erratum in: Nat Neurosci. 2020 Feb;23(2):294. doi: 10.1038/s41593-020-0595-9. Erratum in: Nat Neurosci. 2020 Oct;23(10):1308. doi: 10.1038/s41593-020-0682-y. PMID: 31959936; PMCID: PMC7595134.
- Benador IY, Veliova M, Mahdaviani K, Petcherski A, Wikstrom JD, Assali EA, Acín-Pérez R, Shum M, Oliveira MF, Cinti S, Sztalryd C, Barshop WD, Wohlschlegel JA, Corkey BE, Liesa M, Shirihai OS. Mitochondria Bound to Lipid Droplets Have Unique Bioenergetics, Composition, and Dynamics that Support Lipid Droplet Expansion. Cell Metab. 2018 Apr 3;27(4):869-885.e6. doi: 10.1016/j.cmet.2018.03.003. PMID: 29617645; PMCID: PMC5969538.
- Schott MB, Weller SG, Schulze RJ, Krueger EW, Drizyte-Miller K, Casey CA, McNiven MA. Lipid droplet size directs lipolysis and lipophagy catabolism in hepatocytes. J Cell Biol. 2019 Oct 7;218(10):3320-3335. doi: 10.1083/jcb.201803153. Epub 2019 Aug 7. PMID: 31391210; PMCID: PMC6781454.
- Prentki M, Madiraju SR. Glycerolipid metabolism and signaling in health and disease. Endocr Rev. 2008 Oct;29(6):647-76. doi: 10.1210/er.2008-0007. Epub 2008 Jul 7. PMID: 18606873.
- Veliova M, Ferreira CM, Benador IY, Jones AE, Mahdaviani K, Brownstein AJ, Desousa BR, Acín-Pérez R, Petcherski A, Assali EA, Stiles L, Divakaruni AS, Prentki M, Corkey BE, Liesa M, Oliveira MF, Shirihai OS. Blocking mitochondrial pyruvate import in brown adipocytes induces energy wasting via lipid cycling. EMBO Rep. 2020 Dec 3;21(12):e49634. doi: 10.15252/embr.201949634. Epub 2020 Dec 4. PMID: 33275313; PMCID: PMC7726774.
doi: https://doi.org/10.1242/prelights.38200
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the biochemistry category:
Active flows drive clustering and sorting of membrane components with differential affinity to dynamic actin cytoskeleton
Teodora Piskova
Snake venom metalloproteinases are predominantly responsible for the cytotoxic effects of certain African viper venoms
Daniel Osorno Valencia
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Also in the cell biology category:
Resilience to cardiac aging in Greenland shark Somniosus microcephalus
Theodora Stougiannou
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
Also in the neuroscience category:
Electrophysiological correlates of conscious experiences during sleep: Lucid dreams, sleep paralysis, out-of-body experiences, and false awakenings
uMontreal Neuro preLighters et al.
PPARδ activation in microglia drives a transcriptional response that primes phagocytic function while countering inflammatory activation
Isabel Paine
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
preLists in the biochemistry category:
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
Peer Review in Biomedical Sciences
Communication of scientific knowledge has changed dramatically in recent decades and the public perception of scientific discoveries depends on the peer review process of articles published in scientific journals. Preprints are key vehicles for the dissemination of scientific discoveries, but they are still not properly recognized by the scientific community since peer review is very limited. On the other hand, peer review is very heterogeneous and a fundamental aspect to improve it is to train young scientists on how to think critically and how to evaluate scientific knowledge in a professional way. Thus, this course aims to: i) train students on how to perform peer review of scientific manuscripts in a professional manner; ii) develop students' critical thinking; iii) contribute to the appreciation of preprints as important vehicles for the dissemination of scientific knowledge without restrictions; iv) contribute to the development of students' curricula, as their opinions will be published and indexed on the preLights platform. The evaluations will be based on qualitative analyses of the oral presentations of preprints in the field of biomedical sciences deposited in the bioRxiv server, of the critical reports written by the students, as well as of the participation of the students during the preprints discussions.
| List by | Marcus Oliveira et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
20th “Genetics Workshops in Hungary”, Szeged (25th, September)
In this annual conference, Hungarian geneticists, biochemists and biotechnologists presented their works. Link: http://group.szbk.u-szeged.hu/minikonf/archive/prg2021.pdf
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Also in the cell biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
December in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cell cycle and division 2) cell migration and cytoskeleton 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Matthew Davies et al. |
November in preprints – the CellBio edition
This is the first community-driven preList! A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. Categories include: 1) cancer cell biology 2) cell cycle and division 3) cell migration and cytoskeleton 4) cell organelles and organisation 5) cell signalling and mechanosensing 6) genetics/gene expression
| List by | Felipe Del Valle Batalla et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |
Also in the neuroscience category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |











 (No Ratings Yet)
(No Ratings Yet)